LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible
Does GLP-1 help lower cholesterol? GLP-1 receptor agonists produce modest but meaningful improvements in cholesterol and triglyceride levels, though lipid reduction is not their primary indication. These medications, including semaglutide (Ozempic, Wegovy) and liraglutide (Victoza, Saxenda), typically reduce triglycerides by 10-20% and LDL cholesterol by 2-10%. While these lipid changes are clinically beneficial, GLP-1 agents should complement—not replace—statin therapy for patients requiring significant cholesterol lowering. Their broader cardiovascular benefits, demonstrated in multiple outcome trials, extend beyond lipid effects alone, making them valuable additions to comprehensive cardiometabolic risk management in appropriate patients.
Quick Answer: GLP-1 receptor agonists produce modest cholesterol reductions (LDL-C by 2-10%, triglycerides by 10-20%) but should complement, not replace, statin therapy for lipid management.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications originally developed for type 2 diabetes management that have gained significant attention for their broader metabolic effects. These agents include semaglutide (Ozempic, Wegovy, Rybelsus), dulaglutide (Trulicity), liraglutide (Victoza, Saxenda), among others. Tirzepatide (Mounjaro, Zepbound) is a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, not a pure GLP-1 agent. These medications work by mimicking the action of naturally occurring GLP-1, an incretin hormone released from the intestine in response to food intake.
The primary mechanism of action involves binding to GLP-1 receptors on pancreatic beta cells, which stimulates glucose-dependent insulin secretion and suppresses inappropriate glucagon release. This dual effect helps improve glycemic control without causing significant hypoglycemia in most patients, though the risk increases when combined with insulin or sulfonylureas. Beyond glycemic control, these medications slow gastric emptying, which prolongs satiety and reduces appetite through central nervous system pathways involving the hypothalamus and brainstem.
These medications have demonstrated substantial weight loss effects, with the magnitude varying by agent and dose. Semaglutide 2.4 mg (Wegovy) typically produces ~15% weight reduction, liraglutide 3.0 mg (Saxenda) ~8%, and tirzepatide (Zepbound) ~15-21%. The FDA has approved several agents specifically for chronic weight management in adults with obesity or overweight with at least one weight-related comorbidity. The weight reduction and improved insulin sensitivity contribute to favorable changes across multiple cardiometabolic risk factors.
Most GLP-1 receptor agonists are administered via subcutaneous injection, with dosing frequencies ranging from daily to once weekly depending on the specific agent. Oral semaglutide (Rybelsus) is available for type 2 diabetes treatment. Common adverse effects include gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation, which typically diminish over time with gradual dose titration. Other safety considerations include risk of gallbladder disease, pancreatitis, diabetic retinopathy complications (particularly with rapid A1c reduction), and hypersensitivity reactions. These medications carry a boxed warning for medullary thyroid carcinoma and are contraindicated in patients with personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2.
GLP-1 receptor agonists demonstrate modest but clinically meaningful effects on lipid profiles, though cholesterol reduction is not their primary therapeutic indication. Clinical trial data consistently show that these medications produce small to moderate improvements in several lipid parameters, with the magnitude of effect varying by specific agent and patient population.
The most consistent lipid change observed with GLP-1 therapy is a reduction in triglycerides, typically ranging from 10-20% in clinical studies. Low-density lipoprotein cholesterol (LDL-C) reductions are more modest, generally in the range of 2-10%, though some patients experience more substantial decreases. Total cholesterol typically decreases by 3-8%. High-density lipoprotein cholesterol (HDL-C) changes are variable and generally minimal, with some studies showing slight increases and others showing no significant change. Non-HDL cholesterol and apolipoprotein B may also show modest improvements, which better reflect atherogenic lipoprotein burden.
The lipid-lowering effects of GLP-1 medications appear to be mediated through several mechanisms:
Weight loss: Reduction in body weight and visceral adiposity improves insulin sensitivity and hepatic lipid metabolism
Improved glycemic control: Better glucose regulation reduces hepatic very-low-density lipoprotein (VLDL) production
Potential hepatic effects: Some evidence suggests GLP-1 signaling may influence lipid metabolism, though human data are limited
Reduced postprandial lipemia: Delayed gastric emptying decreases triglyceride excursions after meals
It is important to note that GLP-1 receptor agonists should not be considered primary lipid-lowering agents. According to American Diabetes Association and American College of Cardiology/American Heart Association guidelines, patients with dyslipidemia requiring significant LDL-C reduction will still need statin therapy or other dedicated lipid-lowering medications. For patients with LDL-C ≥190 mg/dL, high-intensity statins are first-line therapy, with consideration of ezetimibe, bempedoic acid, or PCSK9 inhibitors for additional lowering. The lipid improvements with GLP-1 therapy are generally proportional to the degree of weight loss achieved, and individual responses vary considerably.
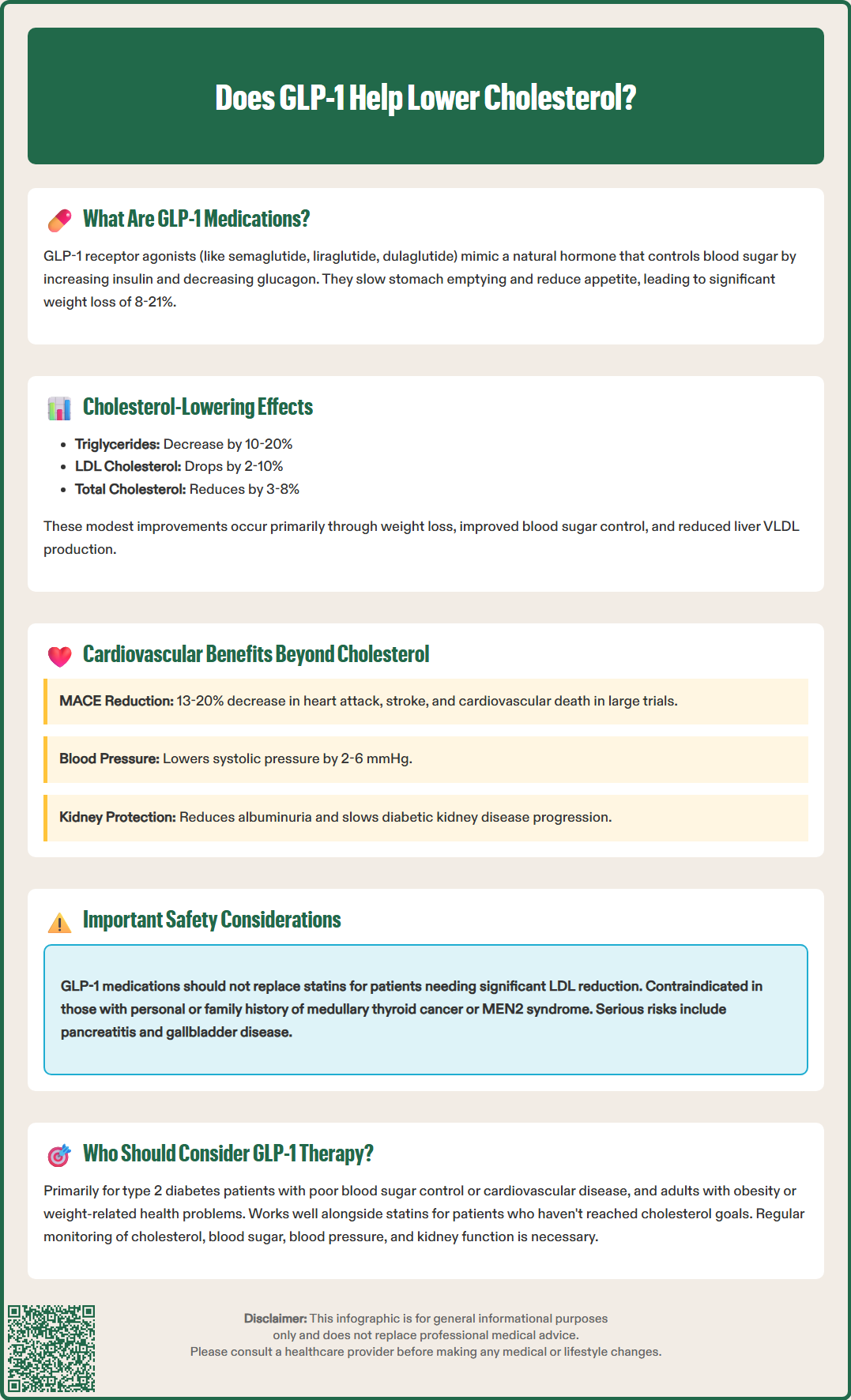
Beyond modest lipid improvements, GLP-1 receptor agonists have demonstrated substantial cardiovascular benefits in large-scale outcome trials, establishing them as important cardioprotective agents for appropriate patients. Multiple cardiovascular outcomes trials (CVOTs) have shown significant reductions in major adverse cardiovascular events (MACE), which typically includes cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
The SELECT trial with semaglutide 2.4 mg demonstrated a 20% reduction in MACE among patients with established cardiovascular disease and overweight or obesity, even in those without diabetes. Similarly, the LEADER trial with liraglutide showed a 13% reduction in MACE in patients with type 2 diabetes and high cardiovascular risk. The REWIND trial with dulaglutide and SUSTAIN-6 with semaglutide also demonstrated cardiovascular benefits. These improvements appear to extend beyond what would be expected from glucose lowering and modest lipid changes alone.
Several mechanisms have been proposed to explain the cardiovascular protection, including:
Blood pressure reduction: GLP-1 agents typically lower systolic blood pressure by 2-6 mmHg through natriuretic effects and improved endothelial function
Anti-inflammatory effects: Reduction in systemic inflammation markers including C-reactive protein and interleukin-6
Improved endothelial function: Enhanced nitric oxide availability and reduced oxidative stress
Potential vascular effects: Emerging research suggests possible benefits on vascular function, though more human data are needed
Additionally, GLP-1 medications have shown benefits in reducing albuminuria and slowing progression of diabetic kidney disease. The FLOW trial with semaglutide demonstrated significant reductions in kidney outcomes in patients with type 2 diabetes and chronic kidney disease. These renal benefits independently contribute to cardiovascular risk reduction, though SGLT2 inhibitors remain foundational therapy for heart failure and CKD when appropriate.
The American College of Cardiology, American Heart Association, and American Diabetes Association now recommend GLP-1 receptor agonists with proven cardiovascular benefit for patients with type 2 diabetes and established atherosclerotic cardiovascular disease, regardless of baseline hemoglobin A1c levels.
While GLP-1 receptor agonists should not be prescribed solely for cholesterol lowering, certain patient populations may derive particular benefit from their lipid and broader cardiometabolic effects. Clinical decision-making should consider the totality of cardiovascular risk factors rather than focusing exclusively on lipid parameters.
Primary candidates for GLP-1 therapy include:
Patients with type 2 diabetes and inadequate glycemic control despite metformin therapy
Individuals with type 2 diabetes and established atherosclerotic cardiovascular disease
Patients with type 2 diabetes and multiple cardiovascular risk factors (hypertension, dyslipidemia, obesity)
Adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with weight-related comorbidities
Patients with metabolic syndrome who have not achieved adequate risk factor control with lifestyle modification
For patients with dyslipidemia, GLP-1 agents may be particularly valuable when combined with statin therapy in those who have not reached LDL-C goals or who have persistent hypertriglyceridemia despite statin treatment. The complementary mechanisms of action can provide additive cardiovascular risk reduction. Patients with statin intolerance should first consider evidence-based alternatives like ezetimibe, bempedoic acid, PCSK9 inhibitors, or inclisiran before considering GLP-1 therapy primarily for lipid management.
For severe hypertriglyceridemia (≥500 mg/dL), fibrates or prescription omega-3 fatty acids (icosapent ethyl) remain first-line to prevent pancreatitis, though GLP-1 therapy may provide additional benefit in appropriate patients.
Contraindications include personal or family history of medullary thyroid carcinoma, multiple endocrine neoplasia syndrome type 2, and serious hypersensitivity to the medication. Precautions and not-recommended situations include history of pancreatitis, severe gastroparesis or other serious gastrointestinal disease, pregnancy/lactation (discontinue before conception), and type 1 diabetes. Patients should be counseled about gastrointestinal side effects and the importance of gradual dose titration.
Clinicians should emphasize that GLP-1 medications complement but do not replace evidence-based lipid management with statins. Regular monitoring of lipid panels, hemoglobin A1c, blood pressure, and renal function is appropriate for patients on GLP-1 therapy. Referral to endocrinology, cardiology, or lipid specialists may be warranted for complex cases involving multiple cardiovascular risk factors, familial hypercholesterolemia, severe hypertriglyceridemia (≥500 mg/dL), or inadequate response to initial therapy.
No, GLP-1 receptor agonists should not replace statins for cholesterol management. While they produce modest LDL cholesterol reductions of 2-10%, statins remain first-line therapy for patients requiring significant lipid lowering, and GLP-1 agents work best as complementary therapy alongside statins.
GLP-1 receptor agonists typically reduce triglycerides by 10-20% in clinical studies. This effect occurs through weight loss, improved insulin sensitivity, reduced hepatic VLDL production, and decreased postprandial lipemia, though individual responses vary.
Primary candidates include patients with type 2 diabetes and established cardiovascular disease, those with multiple cardiovascular risk factors including dyslipidemia, and adults with obesity or overweight with weight-related comorbidities. Guidelines recommend GLP-1 agents with proven cardiovascular benefit for patients with type 2 diabetes and atherosclerotic cardiovascular disease.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.