LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

How to keep semaglutide cold when traveling requires careful planning to protect this temperature-sensitive medication. Semaglutide (Ozempic, Wegovy) is a GLP-1 receptor agonist that must be refrigerated at 36°F to 46°F (2°C to 8°C) before first use to maintain its effectiveness. Temperature excursions can permanently damage the peptide structure, reducing therapeutic benefits for diabetes management and weight control. Whether traveling domestically or internationally, proper cooling solutions and understanding TSA regulations are essential to ensure your medication remains effective throughout your journey. This guide provides practical strategies for maintaining appropriate storage conditions during all phases of travel.
Quick Answer: Semaglutide must be kept refrigerated at 36°F to 46°F (2°C to 8°C) during travel using medical-grade cooling cases with gel packs or phase-change materials, carried in hand luggage to prevent temperature damage.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management and chronic weight management. As a peptide-based biologic medication, semaglutide requires specific storage conditions to maintain its structural integrity and therapeutic efficacy.
According to FDA-approved prescribing information, unopened Ozempic (semaglutide for diabetes) and Wegovy (semaglutide for weight management) pens must be refrigerated at 36°F to 46°F (2°C to 8°C). Both medications should be stored in their original carton to protect from light.
Exposure to temperatures outside the recommended range can denature the peptide structure, potentially reducing the medication's effectiveness. Once the molecular structure of semaglutide is compromised, the damage cannot be reversed. This means that improperly stored medication may fail to provide adequate glycemic control or weight management benefits, even if it appears visually unchanged.
After first use, Ozempic pens may be stored at room temperature (up to 86°F or 30°C) for up to 56 days. Wegovy, which comes in single-dose pens, has different guidelines - when refrigeration is not possible, Wegovy may be kept at temperatures up to 86°F (30°C) for up to 28 days. Both medications should never be frozen, as freezing causes irreversible damage to the medication.
The Transportation Security Administration (TSA) permits passengers to carry medically necessary liquids, including injectable medications like semaglutide, in carry-on baggage without adhering to the standard 3.4-ounce liquid restriction. Passengers should inform TSA officers at the security checkpoint that they are traveling with refrigerated medication and present it separately for inspection. The medication does not need to be placed in a zip-top bag, and reasonable quantities for the trip duration are allowed.
Semaglutide pens and associated cooling accessories should always be packed in carry-on luggage rather than checked baggage. Cargo holds are not temperature-controlled and can expose medications to freezing temperatures at high altitudes or excessive heat on the tarmac, both of which can permanently damage semaglutide. The FDA prescribing information explicitly states that semaglutide must not be frozen.
Passengers may bring gel ice packs, frozen gel packs, or other cooling elements to keep medication cold during travel. These items are permitted even if partially frozen or slushy, provided they are used to cool medically necessary items. TSA officers may need to inspect cooling containers, so using a clear or easily opened insulated case can expedite security screening. It is advisable to carry a copy of your prescription or a letter from your healthcare provider confirming the medical necessity of the medication, particularly for international travel where customs regulations may vary.
Do not rely on airlines to refrigerate your medication. While some airlines may offer to store medications in galley refrigerators on request, this service is not guaranteed and varies by carrier. Always bring your own reliable cooling solution for your entire journey. The TSA also allows travelers to bring empty sharps disposal containers and unused syringes when accompanied by medication.
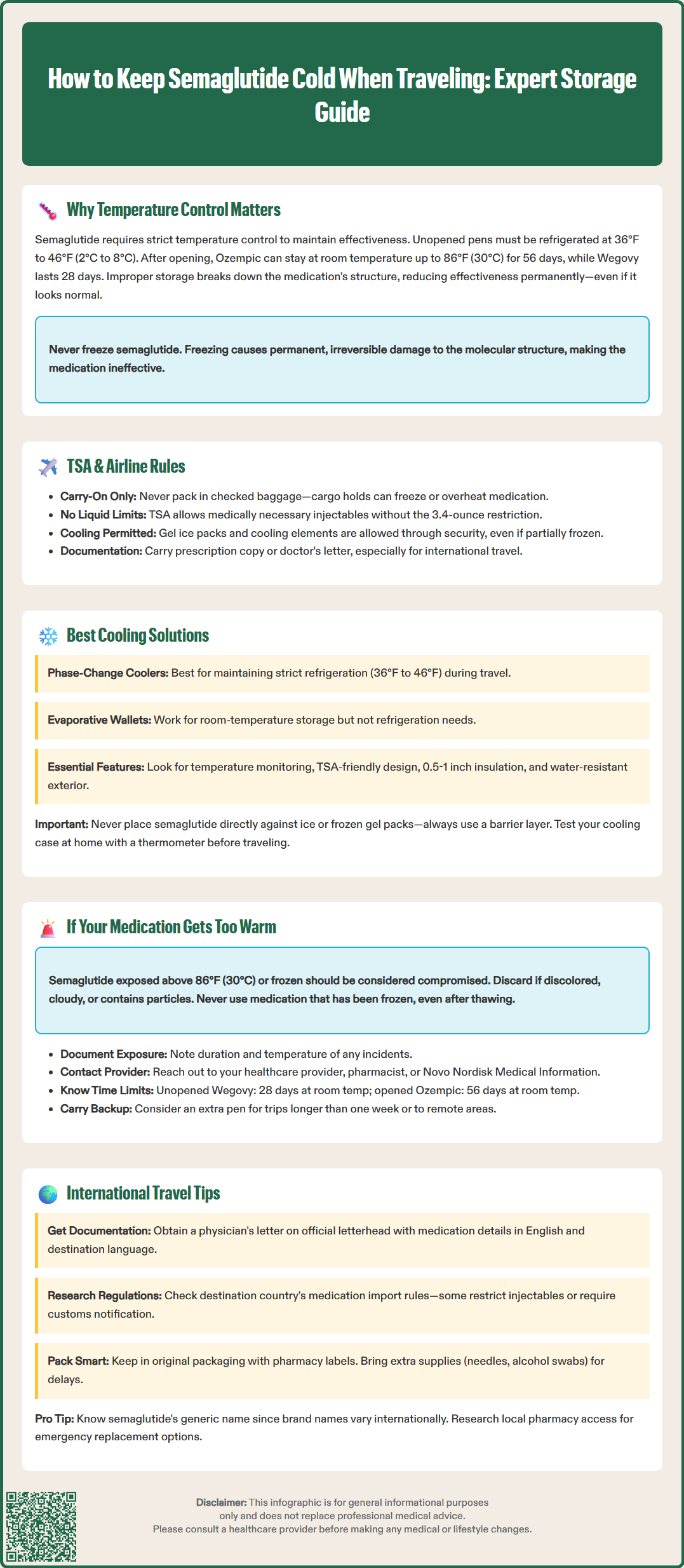
Medical-grade cooling cases designed for injectable medications offer reliable temperature control for semaglutide during travel. These products typically use different cooling technologies to maintain appropriate temperatures for extended periods:
Phase-change material (PCM) coolers: These use special materials that maintain consistent temperatures. For medications requiring refrigeration (36°F to 46°F/2°C to 8°C), look specifically for PCM coolers validated for this temperature range.
Insulated cases with gel packs: These use pre-frozen gel packs and insulation to maintain cool temperatures. Place a barrier between medication and frozen packs to prevent freezing.
Evaporative cooling wallets: Products like FRIO cooling wallets use water-activated evaporative cooling. Note that these typically maintain temperatures below ambient (around 65-79°F/18-26°C) and are more suitable for medications that can be kept at room temperature rather than those requiring strict refrigeration.
Portable medication refrigerators: These require power sources (USB, car adapter) and may be less practical for air travel.
Key features to look for in a cooling case:
Temperature monitoring capability (some cases include built-in thermometers)
TSA-friendly design (easy to open for security inspection)
Adequate insulation (typically 0.5 to 1 inch of foam insulation)
Proper sizing (accommodates your pen(s) without excessive empty space)
Durable, water-resistant exterior
Avoid placing semaglutide directly against ice packs or frozen gel packs, as direct contact with freezing surfaces can cause the medication to freeze. Keep medication in its original carton to protect from light. Before travel, test your cooling solution at home by placing a thermometer inside the case to verify it maintains appropriate temperatures for the expected duration of your journey.
If you suspect your semaglutide has been exposed to temperatures above 86°F (30°C) or if the medication has been frozen, it should be considered potentially compromised. Unfortunately, there is no reliable way to visually determine whether semaglutide has lost potency due to temperature excursions, as the solution typically remains clear and colorless even when degraded. The medication should be inspected for obvious signs of damage, including discoloration, cloudiness, or particulate matter, any of which indicate the medication should not be used.
If temperature excursion occurs during travel, document the circumstances, including estimated duration of exposure and approximate temperature if known. Contact your healthcare provider, pharmacist, or the manufacturer's medical information department as soon as possible to report the incident and seek guidance. For Ozempic and Wegovy, you can contact Novo Nordisk Medical Information for specific advice about temperature excursions.
Remember the product-specific storage allowances:
Unopened Wegovy may be kept at temperatures up to 86°F (30°C) for up to 28 days if refrigeration is not possible
Ozempic, after first use, may be stored at temperatures up to 86°F (30°C) for up to 56 days
Patients should never use semaglutide that has been frozen, even if it has subsequently thawed, as freezing causes irreversible protein denaturation. If you must discard compromised medication while traveling, contact your pharmacy or insurance provider to arrange for replacement. Insurance coverage for emergency medication replacement varies by plan, so check your specific policy details before traveling. Some plans may require prior authorization for replacement.
To prevent therapeutic gaps, some clinicians recommend patients carry a backup pen when traveling, particularly for trips longer than one week or to remote destinations where pharmacy access may be limited. This redundancy provides security against loss, damage, or temperature excursion affecting your primary medication supply.
International travel with semaglutide requires additional planning beyond domestic trips due to varying customs regulations, potential language barriers, and differences in medication availability. Before departure, obtain a letter from your prescribing physician on official letterhead that includes your name, the medication name (both brand and generic), dosage, administration route, and a statement of medical necessity. This documentation should be in English and, if possible, translated into the language of your destination country. Keep medications in their original packaging with pharmacy labels clearly visible.
Research the specific medication import regulations for your destination country well in advance of travel. While semaglutide is widely available internationally, some countries have restrictions on importing injectable medications or require advance notification to customs authorities. The US Department of State and destination country embassy websites provide information on medication import requirements. For extended stays, verify whether your destination country allows you to bring sufficient medication for your entire trip or if you will need to obtain refills locally.
Essential preparation steps for international travel:
Carry extra supplies (additional pen, needles, alcohol swabs) in case of delays
Know the generic name (semaglutide) as brand names vary internationally
Research local pharmacy access at your destination for emergency replacement
Understand time zone adjustments for dosing schedules (if changing your weekly injection day, ensure at least 48 hours have passed since your last dose)
Pack cooling supplies for return journey (ice packs may need replacement)
When flying internationally, be aware that long-haul flights may involve extended periods without access to refrigeration. Plan your cooling strategy accordingly, using high-capacity cooling cases or multiple ice pack sets that can be rotated. Some international airports have medical facilities or pharmacies that may provide ice pack replacement services, though this should not be relied upon. Upon arrival, immediately transfer your medication to refrigerated storage at your accommodation. If hotel mini-bar refrigerators are unreliable or frequently accessed by housekeeping, consider requesting a dedicated small refrigerator or using a portable medication cooler with regular ice replacement throughout your stay.
Yes, TSA permits semaglutide and cooling accessories in carry-on baggage as medically necessary items without the standard 3.4-ounce liquid restriction. Inform TSA officers at security and present the medication separately for inspection.
Semaglutide exposed to temperatures above 86°F (30°C) or frozen may be permanently damaged and lose effectiveness. Contact your healthcare provider or the manufacturer immediately to report the temperature excursion and determine if the medication should be replaced.
Always pack semaglutide in carry-on luggage, never in checked baggage. Cargo holds are not temperature-controlled and can expose the medication to freezing temperatures at altitude or excessive heat on the tarmac, causing irreversible damage.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.