LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Semaglutide (Ozempic, Wegovy) is an FDA-approved GLP-1 receptor agonist for type 2 diabetes and chronic weight management, formulated exclusively for subcutaneous injection into fatty tissue beneath the skin. The FDA labeling explicitly prohibits intramuscular or intravenous administration, as these routes were not studied in clinical trials. Proper injection technique ensures gradual medication absorption, stable drug levels, and minimized adverse effects. Understanding what happens if you inject semaglutide into muscle—and how to avoid this error—is essential for treatment safety and efficacy. This article examines the potential consequences of intramuscular injection, signs of incorrect administration, and evidence-based techniques for proper subcutaneous delivery.
Quick Answer: Semaglutide injected into muscle tissue may cause increased pain and altered absorption, though this route was not studied in clinical trials and is not FDA-approved.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (under the brand name Ozempic) and chronic weight management (Wegovy). The medication is specifically formulated and FDA-approved for subcutaneous injection only—meaning it should be administered into the fatty tissue layer beneath the skin, not into muscle tissue or blood vessels.
According to the FDA-approved prescribing information, semaglutide must be administered subcutaneously into the abdomen, thigh, or upper arm. The upper arm site for Wegovy may require assistance from a caregiver for proper administration. The FDA labeling explicitly states that semaglutide should not be administered intravenously or intramuscularly, as these routes were not studied in clinical trials.
Subcutaneous administration allows for gradual, controlled absorption of the medication into the bloodstream, which helps maintain stable drug levels and minimize adverse effects. The approved injection sites all have adequate subcutaneous tissue for proper drug delivery.
Intramuscular (IM) injection, by contrast, delivers medication directly into muscle tissue, which has a different vascular supply and absorption profile. While IM injections are appropriate for certain medications—such as some vaccines and antibiotics—semaglutide has not been studied or approved for this route of administration. The distinction matters because the depth of injection affects how quickly the drug enters circulation and how the body processes it.
Understanding this difference is crucial for patient safety and treatment efficacy. Healthcare providers should ensure patients receive proper injection technique training before initiating semaglutide therapy to ensure the medication is administered as intended.
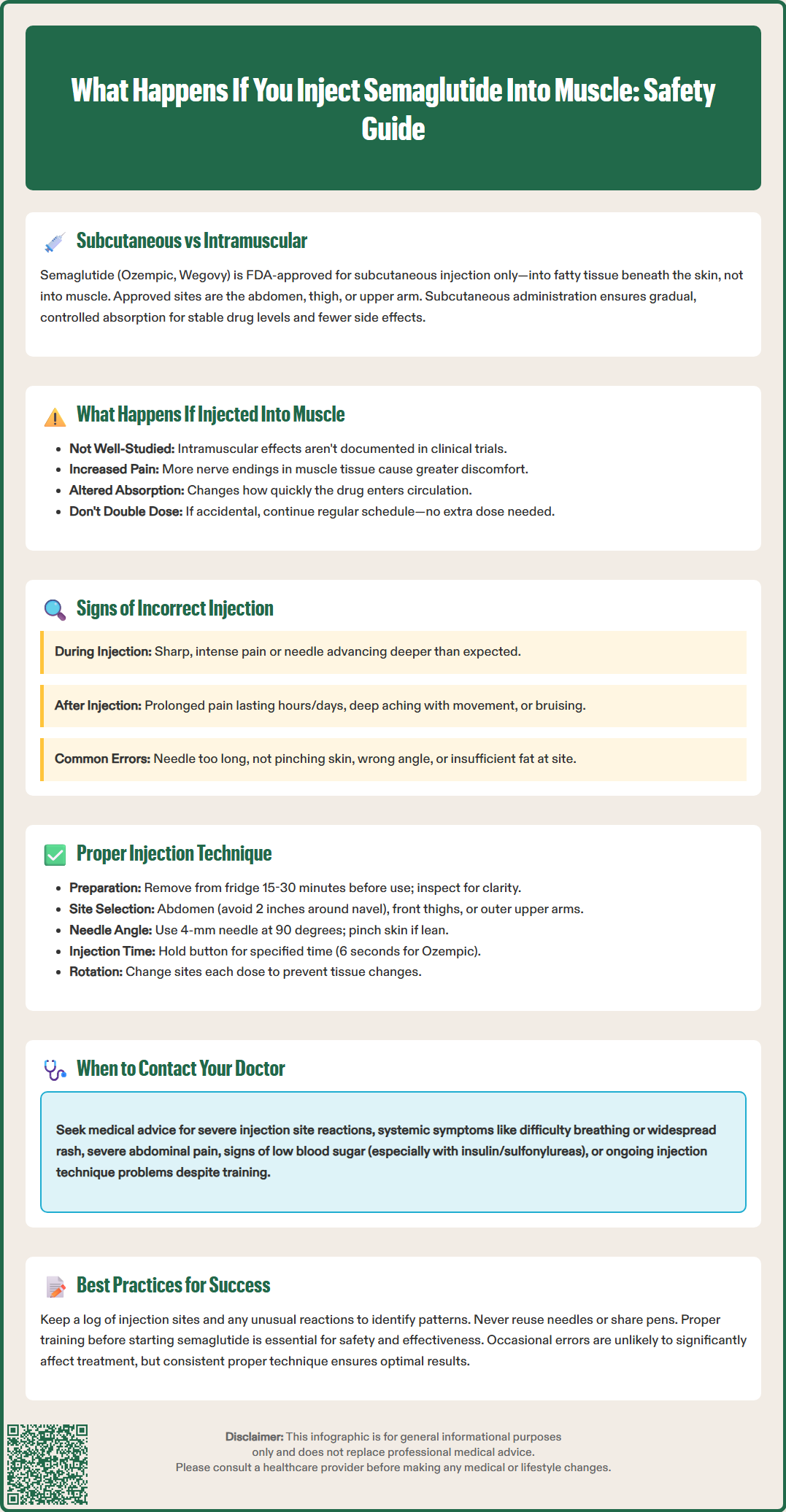
When semaglutide is inadvertently injected into muscle tissue rather than subcutaneous fat, the effects are not well-documented as this route was not studied in clinical trials. The FDA-approved prescribing information specifically indicates semaglutide is for subcutaneous use only.
While the exact consequences of intramuscular injection are not established in clinical literature, pharmacological principles suggest potential differences in absorption. Muscle tissue generally has a richer blood supply than subcutaneous fat, which could theoretically affect how quickly the medication enters the bloodstream. However, without specific studies on intramuscular semaglutide administration, these effects remain uncertain.
Patients may experience increased pain or discomfort at the injection site if the medication is delivered into muscle rather than subcutaneous tissue. Muscle tissue contains more nerve endings than subcutaneous fat, which may contribute to greater injection-related discomfort.
If you suspect you've accidentally injected semaglutide into muscle:
Do not administer an extra dose to compensate
Continue your regular dosing schedule for future injections
Monitor for unusual symptoms but understand that occasional technique errors are unlikely to significantly compromise overall treatment
Pay attention to blood glucose levels, especially if you also take insulin or sulfonylureas, as these combinations increase hypoglycemia risk
Contact your healthcare provider promptly if you experience:
Severe or persistent abdominal pain (potential sign of pancreatitis)
Signs of allergic reaction (rash, swelling, difficulty breathing)
Symptoms of hypoglycemia (if on insulin or sulfonylureas)
Signs of infection at the injection site
While an occasional intramuscular injection is not typically a medical emergency, proper technique for future injections is important for consistent medication delivery and therapeutic effect.
Recognizing the signs of incorrect semaglutide administration can help patients identify technique errors and make necessary adjustments. While some symptoms overlap with normal injection site reactions, certain indicators suggest the medication may have been delivered into muscle rather than subcutaneous tissue.
Physical sensations during injection often provide the first clue. If you experience sharp, intense pain during needle insertion or medication delivery—distinctly different from the mild pressure or pinch of a proper subcutaneous injection—this may indicate deeper penetration than intended. The needle may also advance more deeply than expected before meeting resistance.
Post-injection symptoms that might suggest incorrect administration include:
Increased pain or soreness lasting several hours or days beyond typical injection site discomfort
Deeper aching sensation in the injection area, particularly with movement of the affected limb
Bruising at the injection site (though this can occur with proper technique as well)
Technique factors that increase the risk of injecting too deeply include:
Using a needle that is too long for your body composition
Failing to pinch the skin adequately before injection (especially important for lean individuals)
Injecting at an incorrect angle
Injecting into areas with minimal subcutaneous fat
If you consistently experience unusual discomfort or have concerns about your injection technique, contact your healthcare provider or diabetes educator. They can review your technique, assess whether adjustments are needed, and provide additional training. Maintaining a log of injection sites and any unusual reactions can help identify patterns that warrant technique modification.
Remember that proper technique includes site rotation to prevent tissue changes that could affect absorption. Your healthcare provider can recommend appropriate needle length and injection technique based on your individual needs.
Proper semaglutide injection technique is essential for medication efficacy, comfort, and safety. Following FDA-approved administration guidelines minimizes the risk of intramuscular injection and other complications.
Preparation steps include:
Remove the semaglutide pen from refrigeration 15-30 minutes before injection if desired (for comfort; not required by labeling)
Wash hands thoroughly with soap and water
Inspect the medication for clarity—it should be clear and colorless; do not use if cloudy or discolored
Attach a new needle for each injection (never reuse needles)
For Ozempic: Perform a flow check (priming) before first use of each new pen according to the Instructions for Use
Note: Wegovy single-use pens do not require priming
Site selection and rotation are critical. The FDA-approved injection sites include: abdomen (avoiding a 2-inch radius around the navel), front of thighs, and outer upper arms. Rotate injection sites with each dose to prevent lipohypertrophy (fatty lumps) or lipoatrophy (fat loss), which can affect absorption. Keep a log of injection sites to ensure systematic rotation.
Injection technique to ensure subcutaneous delivery:
Important safety reminders:
Never share pens or needles with others, even if the needle is changed
Use each needle only once and dispose of properly in a sharps container
Store unused pens in the refrigerator according to label instructions
If you miss a dose, follow the specific instructions in your medication's FDA-approved labeling
Do not attempt to administer an extra dose if you suspect improper injection
Special considerations: Upper arm injections with Wegovy often require assistance from a caregiver. Patients who have lost significant weight during treatment may need technique adjustments as their subcutaneous tissue decreases.
If you experience persistent difficulty with self-injection, request a technique review with a diabetes educator, nurse, or pharmacist. Many healthcare systems offer injection training programs, and video demonstrations are available through manufacturer resources.
When to seek medical advice: Contact your healthcare provider if you experience severe injection site reactions (extensive swelling, severe pain, signs of infection), systemic symptoms following injection (difficulty breathing, widespread rash, dizziness), severe or persistent abdominal pain, or consistent problems with injection technique despite training.
No, do not administer an extra dose if you suspect intramuscular injection. Continue your regular dosing schedule for future injections and contact your healthcare provider if you experience unusual symptoms or have concerns about your injection technique.
Most adults should use a 4-mm pen needle at a 90-degree angle to minimize the risk of intramuscular injection. Very lean individuals should pinch the skin firmly before injection to create a fold of subcutaneous tissue.
Signs may include sharp, intense pain during injection, deeper aching sensation lasting hours or days, and increased soreness with movement of the affected limb. If you consistently experience unusual discomfort, contact your healthcare provider for technique review.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.