LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Does tirzepatide get rid of cellulite? This question arises frequently as tirzepatide (Mounjaro, Zepbound) gains recognition for substantial weight loss in clinical trials. While this dual GIP/GLP-1 receptor agonist is FDA-approved for type 2 diabetes and chronic weight management, no clinical evidence supports its use for cellulite reduction. Cellulite involves structural connective tissue changes beneath the skin, not simply excess fat accumulation. Understanding tirzepatide's actual mechanisms, approved indications, and the complex nature of cellulite helps patients and clinicians set realistic expectations about this medication's cosmetic effects.
Quick Answer: Tirzepatide does not get rid of cellulite, as no clinical trials demonstrate direct effects on cellulite reduction despite substantial weight loss achieved with this medication.
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (marketed as Mounjaro) and chronic weight management (marketed as Zepbound). This dual-agonist mechanism distinguishes tirzepatide from single-pathway GLP-1 receptor agonists such as semaglutide.
The medication works through multiple complementary pathways to achieve glycemic control and weight reduction. As a GLP-1 receptor agonist, tirzepatide enhances glucose-dependent insulin secretion from pancreatic beta cells, suppresses inappropriate glucagon release, and slows gastric emptying. These effects collectively improve postprandial glucose excursions and reduce appetite. The GIP receptor agonism may complement these effects, potentially contributing to the medication's pronounced weight loss effects observed in clinical trials.
In the SURMOUNT-1 clinical trial, participants receiving tirzepatide 10 mg or 15 mg weekly achieved mean weight reductions of approximately 19.5% and 20.9% from baseline over 72 weeks, significantly exceeding placebo responses. For chronic weight management, Zepbound is indicated for adults with BMI ≥30 kg/m² or ≥27 kg/m² with at least one weight-related comorbidity.
Common adverse effects include gastrointestinal symptoms—nausea, vomiting, diarrhea, and constipation—which typically diminish with continued use. Serious but rare risks include pancreatitis (requiring immediate medical attention for severe abdominal pain with or without vomiting), gallbladder disease, and acute kidney injury with dehydration. Patients taking insulin or insulin secretagogues may have increased hypoglycemia risk.
Tirzepatide is administered as a once-weekly subcutaneous injection with dose titration starting at 2.5 mg and increasing every 4 weeks through 5 mg, 7.5 mg, 10 mg, 12.5 mg, to a maximum of 15 mg as tolerated. Tirzepatide may decrease the effectiveness of oral contraceptives, particularly after initiation and dose increases; non-oral or backup contraception is recommended for 4 weeks after these changes. The medication is contraindicated during pregnancy and requires prescription and clinical monitoring.
Cellulite, clinically termed gynoid lipodystrophy or edematous fibrosclerotic panniculopathy, describes the dimpled, irregular skin texture commonly observed on the thighs, buttocks, and abdomen. This cosmetic condition affects approximately 80% to 90% of post-pubertal women regardless of body weight, though prevalence and severity increase with age and higher body mass index.
The pathophysiology of cellulite involves structural changes within the subcutaneous tissue architecture. The condition results from herniation of subcutaneous fat into the dermis through weakened or perpendicular fibrous septae that connect skin to underlying fascia. In women, these connective tissue bands typically run perpendicular to the skin surface, creating chambers that allow fat lobules to protrude upward when enlarged or when skin elasticity decreases. Men generally have oblique or crisscrossing septae patterns, which may explain the lower prevalence of visible cellulite in males.
Multiple factors contribute to cellulite development and severity. Hormonal influences play a significant role, with estrogen affecting fat distribution, blood flow, and connective tissue structure. Genetic predisposition determines skin thickness, fat distribution patterns, and connective tissue architecture. Age-related changes include decreased skin elasticity, reduced dermal thickness, and alterations in microcirculation. Lifestyle factors such as physical inactivity, poor dietary habits, and smoking may exacerbate the condition.
Contrary to popular belief, cellulite is not simply excess fat accumulation but rather a complex interaction between adipose tissue, connective tissue structure, microcirculation, and skin characteristics. This multifactorial etiology explains why weight loss alone often provides incomplete resolution of cellulite appearance.
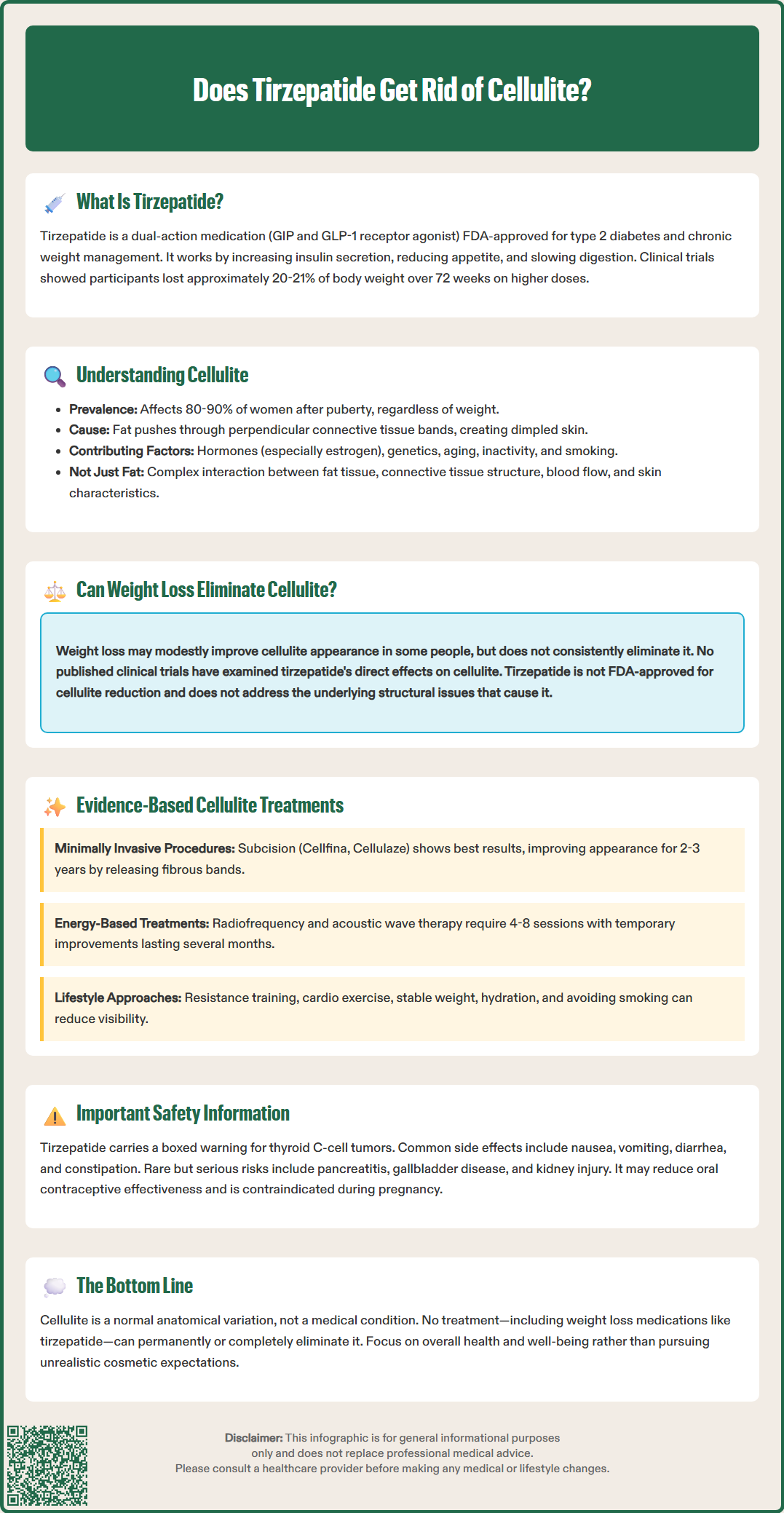
The relationship between weight loss and cellulite reduction remains complex and incompletely understood. While substantial weight loss may improve cellulite appearance in some individuals, there is no official link establishing that weight reduction—whether achieved through lifestyle modification, pharmacotherapy, or bariatric surgery—consistently eliminates cellulite.
Several observational studies suggest modest improvements in cellulite severity scores following significant weight loss. A study published in Plastic and Reconstructive Surgery found that women who lost substantial weight through bariatric surgery experienced some reduction in cellulite severity, though complete resolution was uncommon. The improvement appeared related to decreased adipocyte volume and reduced fat herniation through fibrous septae. However, rapid or extensive weight loss may potentially worsen cellulite appearance in some individuals due to decreased skin elasticity and increased skin laxity.
Regarding tirzepatide specifically, there are no published clinical trials or peer-reviewed studies examining its direct effects on cellulite. While the medication produces substantial weight loss in clinical trials, this does not translate to a specific indication or evidence-based recommendation for cellulite treatment. Tirzepatide is not FDA-approved for cellulite reduction. The structural and connective tissue components of cellulite persist despite fat reduction, and weight loss does not address the fundamental architectural abnormalities underlying the condition.
Patients considering tirzepatide should understand that any potential cosmetic benefits regarding cellulite would be secondary and unpredictable consequences of weight reduction, not primary therapeutic effects. The medication is indicated for glycemic control in type 2 diabetes and for chronic weight management in adults with obesity or overweight with weight-related comorbidities—not for cosmetic concerns. Clinicians should counsel patients that cellulite improvement is not an expected or reliable outcome of tirzepatide therapy.
For patients seeking cellulite reduction, several evidence-based interventions have demonstrated varying degrees of efficacy in clinical studies, though none provide permanent or complete resolution.
Minimally invasive procedures show the most consistent results. Subcision techniques, which involve releasing fibrous septae through small incisions, have demonstrated sustained improvement in cellulite appearance for 2 to 3 years in controlled trials. Laser-assisted subcision devices (such as Cellulaze, FDA-cleared) combine septal release with thermal stimulation of collagen production. Vacuum-assisted precise tissue release (such as Cellfina, FDA-cleared) specifically targets and releases individual dimple-causing septae, with clinical data showing improvement lasting up to 3 years. Common side effects include bruising, pain, and temporary soreness. Collagenase clostridium histolyticum-aaes (QWO) was previously FDA-approved for treating moderate to severe cellulite in the buttocks of adult women but is no longer commercially available in the US market.
Energy-based treatments offer non-invasive alternatives with more modest and temporary results. Radiofrequency devices deliver controlled thermal energy to stimulate collagen remodeling and may temporarily improve skin texture. Acoustic wave therapy (similar to shockwave therapy) aims to improve microcirculation and stimulate connective tissue remodeling, though evidence quality remains limited. These modalities typically require multiple treatment sessions (4-8) and provide temporary improvements lasting several months.
Topical treatments have minimal supporting evidence. Caffeine-containing creams may produce temporary, superficial improvements through mild dehydration effects, but systematic reviews find insufficient evidence for meaningful clinical benefit. Retinoid creams may modestly improve skin thickness over prolonged use but do not address underlying structural abnormalities.
Lifestyle modifications provide supportive benefits. Regular resistance training and cardiovascular exercise improve muscle tone and may reduce fat volume, potentially decreasing cellulite visibility. Maintaining stable body weight prevents fluctuations that may worsen skin laxity. Adequate hydration and smoking cessation support overall skin health.
Clinicians should counsel patients that cellulite represents a normal anatomical variant rather than a medical condition requiring treatment. For those seeking intervention, referral to board-certified dermatologists or plastic surgeons experienced in cellulite treatments ensures appropriate patient selection and realistic expectation-setting. Patients should be advised that no treatment—including weight loss medications like tirzepatide—reliably eliminates cellulite, and combination approaches may provide optimal cosmetic outcomes for motivated individuals.
While substantial weight loss may modestly improve cellulite appearance in some individuals, no clinical evidence demonstrates that tirzepatide specifically reduces cellulite. The structural connective tissue abnormalities underlying cellulite persist despite fat reduction, and weight loss effects on cellulite remain unpredictable and inconsistent.
Tirzepatide is FDA-approved for type 2 diabetes management as Mounjaro and for chronic weight management as Zepbound in adults with BMI ≥30 kg/m² or ≥27 kg/m² with weight-related comorbidities. It is not approved for cellulite reduction or any cosmetic indications.
Evidence-based cellulite treatments include minimally invasive procedures such as subcision techniques and vacuum-assisted precise tissue release (Cellfina), which demonstrate sustained improvement for 2-3 years in clinical trials. Energy-based treatments like radiofrequency provide temporary, modest results, while topical treatments have minimal supporting evidence.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.