LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Allulose, a rare sugar with minimal calories, has gained attention for its potential to stimulate glucagon-like peptide-1 (GLP-1), an incretin hormone crucial for blood sugar regulation and appetite control. While preliminary research suggests allulose may trigger modest GLP-1 secretion, the specific dosage required for meaningful effects remains unclear. Most human studies have examined doses between 5 and 10 grams, though results vary considerably among individuals. Understanding the relationship between allulose intake and GLP-1 response requires careful consideration of current evidence, FDA guidelines, and how this sweetener compares to established diabetes management strategies.
Quick Answer: Research suggests 5 to 10 grams of allulose may produce modest, transient increases in GLP-1 levels, though no minimum effective dose has been established and individual responses vary considerably.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Allulose, also known as D-psicose, is a rare sugar that occurs naturally in small quantities in foods such as wheat, figs, and raisins. Structurally similar to fructose but with a different molecular configuration, allulose provides approximately 0.4 calories per gram compared to the 4 calories per gram found in regular sugar. The FDA has recognized allulose as Generally Recognized as Safe (GRAS) and under enforcement discretion permits its exclusion from total and added sugars labeling, though it still counts toward Total Carbohydrate on nutrition labels. This regulatory approach reflects allulose's minimal caloric contribution and negligible impact on blood glucose levels.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by enteroendocrine L-cells in the intestinal mucosa in response to nutrient intake. GLP-1 plays a crucial role in glucose homeostasis by stimulating insulin secretion in a glucose-dependent manner, suppressing glucagon release, slowing gastric emptying, and promoting satiety. These mechanisms have made GLP-1 receptor agonists valuable therapeutic agents for type 2 diabetes and obesity management.
Emerging research suggests that allulose may stimulate GLP-1 secretion, though the mechanisms remain hypothetical. Some preliminary studies suggest allulose might interact with sweet taste receptors (T1R2/T1R3) or other nutrient-sensing mechanisms on enteroendocrine cells. Unlike what was previously thought, human studies indicate that most ingested allulose is absorbed in the small intestine and rapidly excreted unchanged in the urine, with only a minority reaching the colon. The clinical significance of allulose-induced GLP-1 secretion and the specific dosages required to achieve meaningful physiological effects remain areas of active investigation with limited conclusive evidence.
Current research on allulose and GLP-1 secretion is limited, with most studies conducted in animal models or small human trials. A study published in the journal Nutrients examined the effects of allulose on postprandial glucose and hormone responses in healthy adults. Participants who consumed 5 grams of allulose with a carbohydrate load demonstrated modest increases in GLP-1 levels compared to control groups, though the magnitude of this effect varied considerably among individuals.
Another investigation involving 7.5 grams of allulose administered before a meal showed trends toward elevated GLP-1 concentrations, but these findings did not consistently reach statistical significance across all measured time points. It's important to note that in most human studies, GLP-1 was measured as a secondary or exploratory endpoint rather than the primary outcome, limiting the strength of conclusions that can be drawn.
The variability in results suggests that individual metabolic factors, baseline insulin sensitivity, and other physiological variables may influence the GLP-1 response to allulose. Importantly, these studies have not established a clear dose-response relationship, and there is no consensus on a minimum effective dose for clinically meaningful GLP-1 stimulation.
Doses ranging from 5 to 10 grams have been most commonly studied in human trials, though these investigations primarily focused on glycemic control rather than GLP-1 secretion as a primary endpoint. Higher doses (up to 15 grams per serving) have been evaluated for safety and tolerability but have not been systematically assessed for their effects on incretin hormone secretion. The GLP-1 elevations observed with allulose consumption are generally small and transient compared to those achieved with pharmaceutical GLP-1 receptor agonists such as semaglutide or liraglutide, which produce sustained and pharmacologically significant increases in GLP-1 activity.
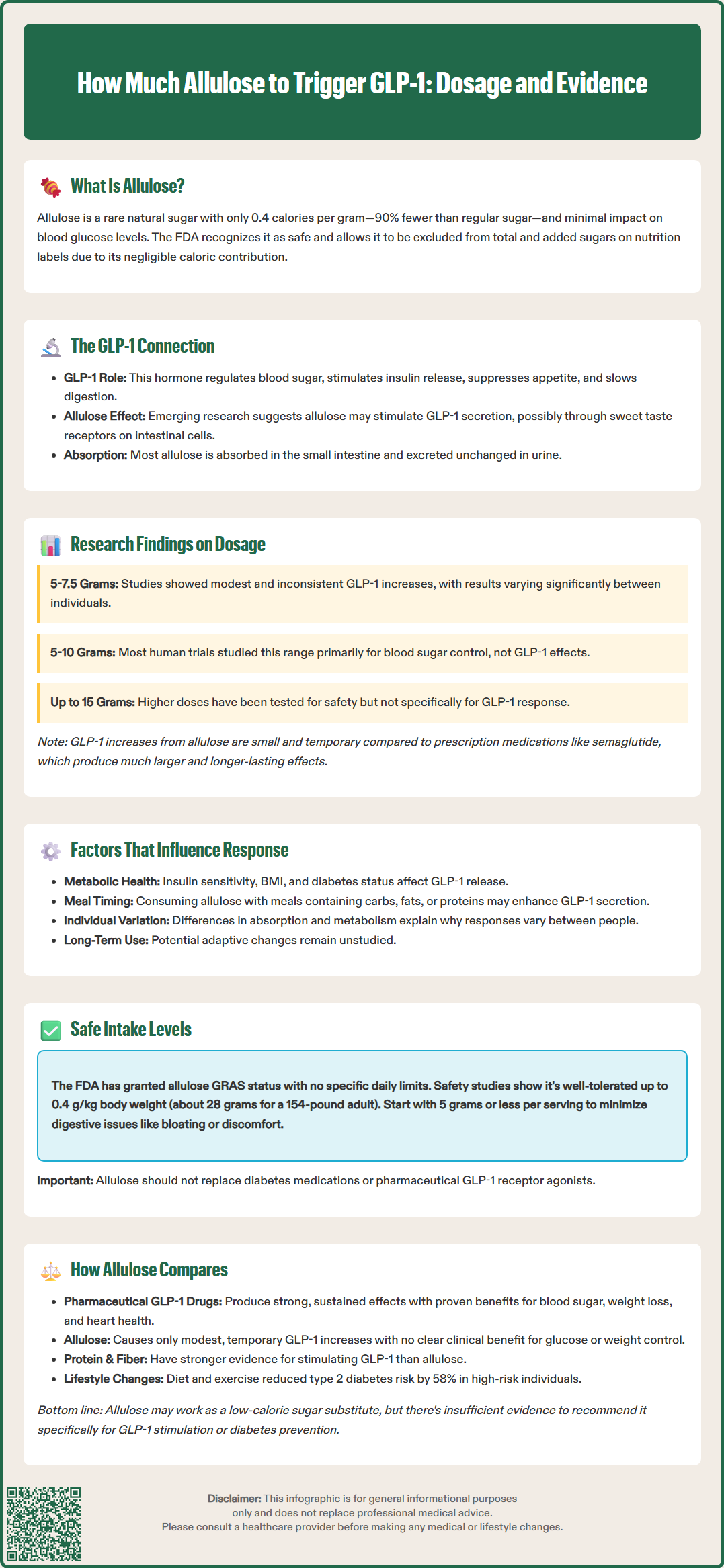
Multiple physiological and contextual factors may modulate the GLP-1 response to allulose consumption. Individual metabolic characteristics, including baseline insulin sensitivity, body mass index, and the presence of prediabetes or type 2 diabetes, appear to influence incretin hormone secretion patterns. Some evidence suggests that individuals with impaired glucose tolerance may exhibit different GLP-1 responses to dietary stimuli compared to metabolically healthy individuals, though specific data regarding allulose in this context remain limited.
The timing and composition of meals consumed with allulose likely affect its impact on GLP-1 secretion. Allulose consumed with carbohydrate-containing meals may interact with glucose-sensing mechanisms in enteroendocrine cells, potentially enhancing or modifying the incretin response. The macronutrient composition of accompanying foods—particularly the presence of fats and proteins, which are known GLP-1 secretagogues—may create additive or synergistic effects that are difficult to isolate in clinical studies.
Gut microbiome composition represents another potentially important variable, though its specific role in allulose metabolism requires further investigation. Since most ingested allulose is absorbed and excreted in urine, with only a fraction reaching the colon, the direct microbiome effects may be less significant than initially hypothesized. Individual differences in absorption and metabolism could contribute to the variability observed in GLP-1 responses across study participants. Additionally, chronic versus acute allulose consumption may produce different effects, as repeated exposure could lead to adaptive changes in gut hormone secretion patterns, though longitudinal data addressing this question are currently lacking.
The FDA has granted allulose GRAS status based on comprehensive safety evaluations, and manufacturers may use it as a food ingredient without specific quantity restrictions beyond good manufacturing practices. The FDA does not provide specific recommended daily intake limits for allulose, as it does for some other food additives. Under FDA guidance, allulose contributes to Total Carbohydrate on nutrition labels but is excluded from Total and Added Sugars, with an assigned caloric value of 0.4 kcal/g.
Safety studies have evaluated single doses up to 0.4 grams per kilogram of body weight (approximately 28 grams for a 70-kilogram adult) without serious adverse effects, though gastrointestinal symptoms represent the primary tolerability concern.
The most commonly reported adverse effects associated with allulose consumption are gastrointestinal in nature, including bloating, abdominal discomfort, and diarrhea. These symptoms are dose-dependent and typically occur at intakes exceeding 10 to 15 grams per serving, particularly in individuals unaccustomed to consuming this sweetener. The mechanism underlying these effects relates to individual tolerance and potential osmotic effects in some consumers.
For individuals considering allulose consumption with the goal of potentially influencing GLP-1 secretion, a cautious approach is warranted. Starting with lower doses (5 grams or less per serving) and gradually increasing intake while monitoring for gastrointestinal tolerance represents a prudent strategy. Patients with diabetes should be advised that while allulose does not significantly raise blood glucose, it should not replace established diabetes management strategies, including prescribed medications, dietary modifications, and regular monitoring. There is no evidence to support using allulose as a substitute for pharmaceutical GLP-1 receptor agonists in patients for whom these medications are indicated.
When evaluating allulose as a potential GLP-1 stimulator, it is essential to contextualize its effects relative to other dietary and pharmaceutical approaches. Pharmaceutical GLP-1 receptor agonists such as semaglutide, dulaglutide, and liraglutide produce sustained, pharmacologically significant elevations in GLP-1 activity, resulting in clinically meaningful improvements in glycemic control, weight reduction, and cardiovascular outcomes in patients with type 2 diabetes. These medications are supported by extensive randomized controlled trial data and are recommended by the American Diabetes Association (ADA) as preferred agents for many patients with type 2 diabetes, particularly those with established cardiovascular disease or chronic kidney disease.
In contrast, the GLP-1 elevations associated with allulose consumption appear modest and transient, with unclear clinical significance for glucose management or weight control. Other dietary approaches may stimulate GLP-1 secretion more reliably, including consumption of protein-rich meals, dietary fiber (particularly viscous fibers), and certain fermentable carbohydrates that promote short-chain fatty acid production in the colon. These dietary strategies have more robust evidence supporting their metabolic benefits compared to allulose.
For patients seeking non-pharmaceutical approaches to enhance incretin function, comprehensive lifestyle modifications remain the foundation of evidence-based care. The Diabetes Prevention Program demonstrated that intensive lifestyle intervention, including dietary changes and physical activity, reduced the incidence of type 2 diabetes by 58% over three years in high-risk individuals. While allulose may serve as a useful low-calorie sweetener alternative for individuals seeking to reduce sugar intake, there is insufficient evidence to recommend it specifically for GLP-1 stimulation or diabetes prevention. Patients with diabetes or prediabetes should work with healthcare providers to develop individualized treatment plans based on established guidelines rather than relying on emerging dietary supplements or sweeteners with limited clinical evidence.
Current research has not established a minimum effective dose, though studies most commonly examine 5 to 10 grams of allulose. Individual responses vary considerably, and the GLP-1 elevations observed are generally modest and transient compared to pharmaceutical GLP-1 medications.
No, allulose should not replace pharmaceutical GLP-1 receptor agonists prescribed for diabetes. The GLP-1 increases from allulose are small and temporary, while medications like semaglutide produce sustained, clinically significant effects supported by extensive clinical trial data.
The primary side effects are gastrointestinal symptoms including bloating, abdominal discomfort, and diarrhea, typically occurring at doses exceeding 10 to 15 grams per serving. Starting with lower doses (5 grams or less) and gradually increasing intake can help minimize these effects.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.