LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Lipo C and tirzepatide represent two distinct approaches to weight management that patients increasingly inquire about using together. Tirzepatide is an FDA-approved dual GIP/GLP-1 receptor agonist proven effective for type 2 diabetes and chronic weight management, while Lipo C refers to unapproved compounded lipotropic injections containing methionine, inositol, choline, and vitamin B12. No clinical trials have evaluated their combined use, and major US guidelines do not recommend lipotropic injections. Understanding the evidence, safety considerations, and lack of proven synergistic benefit is essential for informed clinical decision-making when patients consider combining these treatments.
Quick Answer: No clinical evidence supports combining Lipo C lipotropic injections with tirzepatide, and this combination is not endorsed by FDA or clinical guidelines.
Lipo C refers to lipotropic injections containing a combination of compounds intended to support fat metabolism and liver function. These formulations typically include methionine, inositol, choline, and cyanocobalamin (vitamin B12), often abbreviated as MIC-B12. Lipotropic agents are marketed primarily through wellness clinics and weight management programs as adjuncts to diet and exercise. Methionine is an essential amino acid involved in lipid metabolism, inositol participates in cellular signaling, and choline supports hepatic lipid export. Vitamin B12 is added for its role in energy metabolism. These injections are unapproved compounded products that have not been reviewed by the FDA for safety, effectiveness, or quality. Controlled evidence demonstrating clinically meaningful weight loss from these injections is lacking.
Tirzepatide is an FDA-approved prescription medication marketed as Mounjaro for type 2 diabetes mellitus and Zepbound for chronic weight management in adults with BMI ≥30 kg/m², or ≥27 kg/m² with at least one weight-related comorbidity. It functions as a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. By activating both incretin pathways, tirzepatide enhances insulin secretion in a glucose-dependent manner, suppresses glucagon release, delays gastric emptying, and reduces appetite through central nervous system effects. Tirzepatide is not indicated for type 1 diabetes. Clinical trials have demonstrated substantial glycemic control improvements and weight loss averaging 15-21% of body weight at higher doses. Tirzepatide is administered as a once-weekly subcutaneous injection, with dosing typically initiated at 2.5 mg and increased by 2.5 mg every 4 weeks up to a maintenance dose of 5-15 mg based on tolerability and therapeutic response. Tirzepatide is contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2, and should not be used during pregnancy due to potential fetal harm.
There is no official clinical guidance regarding the concurrent use of lipotropic injections with tirzepatide, as Lipo C formulations are unapproved compounded products that have not been reviewed by the FDA. Major US clinical guidelines for diabetes and obesity management do not include recommendations for lipotropic injections. The absence of pharmacokinetic or pharmacodynamic interaction studies means healthcare providers must rely on theoretical considerations and the known pharmacology of each component when patients inquire about combination use.
From a mechanistic perspective, the individual components of Lipo C injections are unlikely to directly interfere with tirzepatide's receptor binding or metabolic effects. Methionine, inositol, and choline are nutritional compounds involved in separate biochemical pathways from incretin receptor signaling. Vitamin B12 supplementation is generally considered safe across diverse medication regimens. However, the lack of standardized formulation for lipotropic injections presents a significant concern—compounding pharmacies may include additional ingredients such as L-carnitine, lidocaine, other B vitamins, or amino acids that have not been evaluated in combination with GLP-1/GIP agonists.
Patient safety considerations include the potential for injection site reactions when administering multiple subcutaneous injections, particularly if proper site rotation is not maintained. Additionally, patients using both treatments may attribute weight loss or adverse effects to the wrong agent, complicating clinical assessment. Healthcare providers should document all supplements and compounded preparations patients are using (including full ingredient lists and lot numbers), counsel on proper injection technique and site rotation, and monitor for unexpected adverse effects. Any decision to use lipotropic injections alongside tirzepatide should involve informed discussion about the absence of safety data and the unregulated nature of compounded products. Patients should be advised to source any compounded products from reputable pharmacies that follow USP <797> sterile compounding standards. Unexpected adverse events should be reported to the FDA MedWatch program.
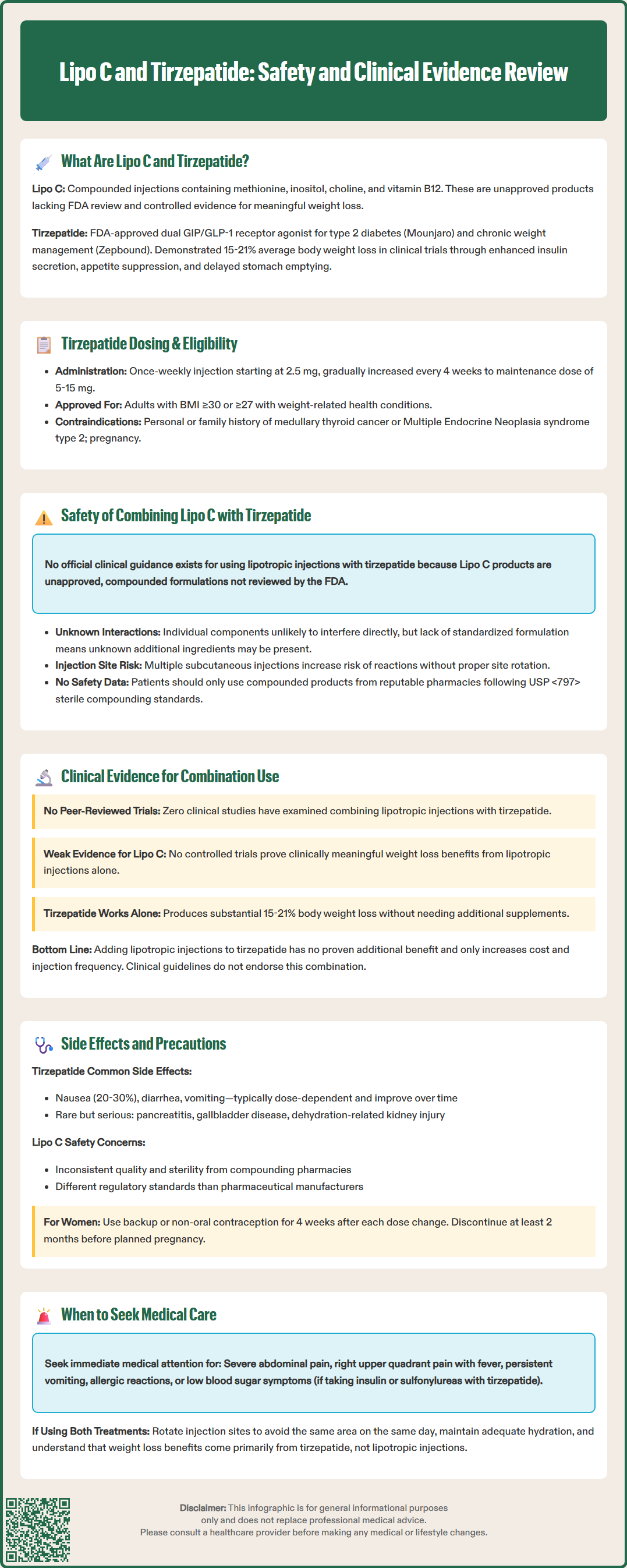
No peer-reviewed clinical trials have evaluated the combined use of lipotropic injections and tirzepatide for weight management or metabolic outcomes. The evidence base for tirzepatide as monotherapy is robust, with pivotal trials including SURMOUNT-1 demonstrating significant weight reduction in adults with obesity, and SURPASS trials establishing glycemic efficacy in type 2 diabetes. These studies did not include lipotropic injections as part of the intervention protocols, and participants were typically excluded if using investigational weight loss compounds.
The evidence supporting lipotropic injections themselves remains limited and of low quality. While individual components like choline have established roles in hepatic lipid metabolism, controlled trials demonstrating clinically meaningful weight loss from MIC-B12 injections are lacking. Most available data consists of observational reports from weight loss clinics where multiple interventions (caloric restriction, exercise programs, behavioral counseling) are implemented simultaneously, making it impossible to isolate the contribution of lipotropic injections.
Given tirzepatide's demonstrated efficacy—with weight loss exceeding that achieved with other approved medications—there is no evidence of additional benefit from adding lipotropic injections, and this combination is not endorsed by clinical guidelines. The substantial weight reduction observed with tirzepatide monotherapy (15-21% body weight in clinical trials) suggests that any perceived benefits likely reflect tirzepatide's pharmacological effects rather than synergistic action. Patients considering combination approaches should understand that this represents an unproven strategy that increases cost and injection burden without documented benefit. Healthcare providers should emphasize evidence-based interventions aligned with current clinical guidelines from organizations such as the American Diabetes Association, American Association of Clinical Endocrinology, and American Gastroenterological Association.
Tirzepatide's adverse effect profile is well-characterized from extensive clinical trial data. The most common side effects are gastrointestinal, including nausea (occurring in 20-30% of patients), diarrhea, vomiting, constipation, and abdominal discomfort. These effects are typically dose-dependent, most pronounced during dose escalation, and tend to diminish over time. Serious but rare adverse effects include pancreatitis, gallbladder disease, acute kidney injury (usually secondary to dehydration from gastrointestinal symptoms), and hypoglycemia when combined with insulin or sulfonylureas. The FDA label includes a boxed warning regarding thyroid C-cell tumors observed in rodent studies, contraindicating use in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2.
Important tirzepatide warnings include potential reduced efficacy of oral contraceptives during initiation and dose increases; patients should use backup or non-oral contraception for 4 weeks after each dose change. Tirzepatide may cause fetal harm and should be discontinued at least 2 months before a planned pregnancy. Renal function should be monitored if significant gastrointestinal symptoms or dehydration occur.
Lipotropic injections may cause local injection site reactions including pain, redness, swelling, or bruising. Allergic reactions to individual components are possible, though uncommon. High-dose methionine supplementation has theoretical concerns regarding homocysteine elevation, though this is unlikely at typical lipotropic injection doses. The primary safety concern with compounded lipotropic preparations is variability in formulation quality and sterility, as compounding pharmacies operate under different regulatory standards than pharmaceutical manufacturers.
When patients use both treatments concurrently, several precautions are warranted:
Injection site management: Rotate sites systematically and avoid administering both injections in the same anatomical area on the same day
Gastrointestinal monitoring: Tirzepatide's GI effects may be misattributed to lipotropic injections, potentially leading to inappropriate dose adjustments
Hydration emphasis: Ensure adequate fluid intake given tirzepatide's potential to cause dehydration through GI effects
Medication adjustments: Consider reducing doses of insulin or sulfonylureas when initiating tirzepatide to mitigate hypoglycemia risk
Realistic expectations: Counsel patients that weight loss benefits derive primarily from tirzepatide's pharmacological action
Cost considerations: Lipotropic injections represent additional out-of-pocket expense without proven incremental benefit
Patients should seek immediate medical attention for severe or persistent abdominal pain (possible pancreatitis), right upper quadrant pain with fever or jaundice (gallbladder disease), signs of allergic reaction, persistent vomiting leading to dehydration, or symptomatic hypoglycemia if on insulin/sulfonylureas. Regular follow-up should include assessment of weight loss trajectory, tolerability, medication adherence, and renal function if significant GI symptoms occur. Healthcare providers should maintain open communication about all treatments patients are using and provide evidence-based guidance prioritizing interventions with established safety and efficacy profiles.
There is no official clinical guidance on combining these treatments, as Lipo C formulations are unapproved compounded products not reviewed by the FDA. While direct drug interactions are unlikely based on known mechanisms, the lack of safety studies and standardized formulations means healthcare providers cannot definitively assess safety or benefit.
No clinical trials demonstrate additional weight loss benefit from adding lipotropic injections to tirzepatide. Tirzepatide monotherapy produces substantial weight reduction in clinical trials, and any perceived benefits from combination use likely reflect tirzepatide's pharmacological effects rather than synergistic action.
Primary concerns include increased injection site reactions from multiple subcutaneous injections, difficulty attributing adverse effects to the correct agent, additional cost without proven benefit, and potential quality variability in compounded lipotropic products. Proper injection site rotation and monitoring for tirzepatide's gastrointestinal effects remain essential.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.