LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Semaglutide interactions with other drugs are an important consideration for patients using this GLP-1 receptor agonist for type 2 diabetes or weight management. While semaglutide has minimal direct pharmacokinetic interactions, its effects on gastric emptying and blood sugar control can influence how other medications work in your body. Understanding these interactions helps prevent complications such as hypoglycemia when combined with insulin or sulfonylureas, ensures proper absorption of medications like levothyroxine, and optimizes timing for oral semaglutide (Rybelsus). This guide explains clinically significant drug interactions, monitoring requirements, and when to consult your healthcare provider.
Quick Answer: Semaglutide has minimal direct drug interactions but may increase hypoglycemia risk when combined with insulin or sulfonylureas, and oral semaglutide can affect absorption of medications like levothyroxine.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for managing type 2 diabetes mellitus and chronic weight management. It works by mimicking the action of endogenous GLP-1, a hormone that stimulates insulin secretion in a glucose-dependent manner, suppresses glucagon release, slows gastric emptying, and promotes satiety through central nervous system pathways. These mechanisms collectively improve glycemic control and facilitate weight reduction.
Semaglutide is available in two formulations: injectable (Ozempic, Wegovy) and oral (Rybelsus). Understanding potential drug interactions with semaglutide is clinically important for several reasons. While semaglutide's effect on gastric emptying can theoretically alter the absorption of orally administered drugs, FDA studies have found no clinically relevant pharmacokinetic interactions with several commonly tested medications. When used alongside other glucose-lowering agents, semaglutide may increase the risk of hypoglycemia, necessitating dose adjustments of concomitant medications. Additionally, certain drug combinations may amplify adverse effects such as gastrointestinal disturbances, which are common with GLP-1 receptor agonists.
Semaglutide does not undergo significant cytochrome P450 metabolism and has minimal direct pharmacokinetic interactions. The FDA-approved prescribing information emphasizes that healthcare providers should evaluate all concurrent medications when initiating semaglutide therapy. Patients taking multiple medications, especially those with narrow therapeutic indices, warrant careful monitoring to optimize therapeutic outcomes and minimize adverse events. For oral semaglutide (Rybelsus), specific timing requirements apply—it must be taken first thing in the morning on an empty stomach with no more than 4 ounces of plain water, at least 30 minutes before food, beverages, or other oral medications.
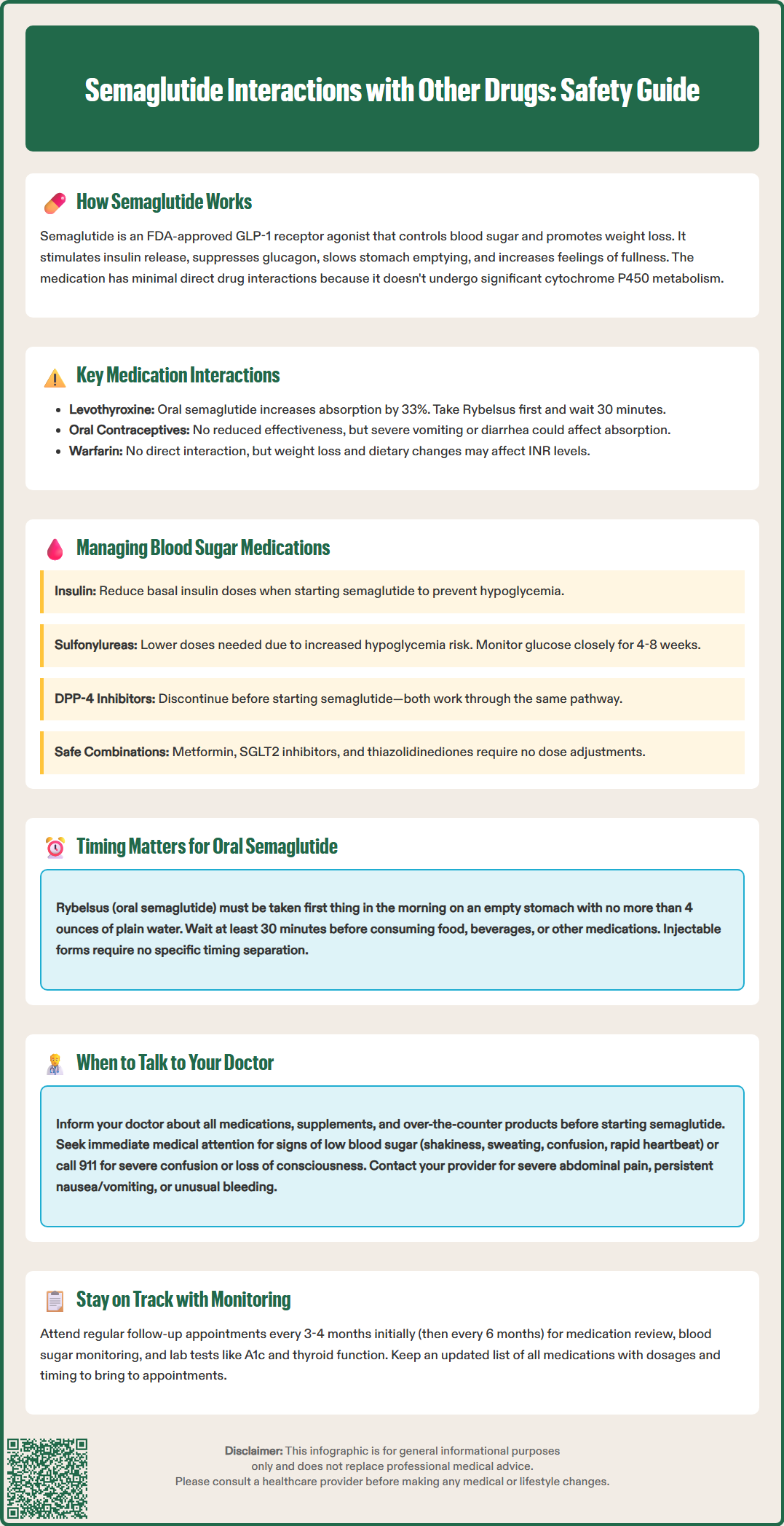
Several medication classes require consideration when prescribing semaglutide due to potential pharmacodynamic or absorption-related interactions. Oral medications with narrow therapeutic indices represent a primary concern. For levothyroxine, oral semaglutide (Rybelsus) may increase thyroxine exposure by approximately 33%. Patients taking both medications should follow Rybelsus timing instructions (taking Rybelsus first, then waiting at least 30 minutes before other oral medications) and have thyroid function tests monitored. For injectable semaglutide formulations, no specific timing separation is required based on current evidence.
Oral contraceptives have been studied with semaglutide, and FDA data show no clinically relevant reduction in contraceptive efficacy. The FDA label does not recommend routine use of backup contraception methods. However, patients should be counseled that severe vomiting or diarrhea (potential side effects when starting semaglutide) could potentially affect oral contraceptive absorption, as with any significant gastrointestinal illness.
Warfarin and other anticoagulants have shown no direct pharmacokinetic interaction or clinically significant effect on INR in FDA studies with semaglutide. However, changes in dietary intake, body weight, and gastrointestinal function associated with semaglutide therapy may indirectly affect warfarin's anticoagulant effect. INR monitoring should be individualized based on clinical factors, with particular attention during significant weight loss or dietary changes.
Time-sensitive medications may require individualized planning. For oral semaglutide (Rybelsus), the requirement to take it at least 30 minutes before other oral medications is critical. For injectable semaglutide formulations, there is no evidence-based recommendation for specific timing separation from other medications. Healthcare providers should develop individualized plans for patients taking medications with narrow therapeutic indices or time-dependent absorption requirements.
The concurrent use of semaglutide with other antidiabetic agents requires careful clinical management to balance glycemic control against hypoglycemia risk. Insulin therapy represents a significant interaction concern. When semaglutide is added to existing insulin regimens, the risk of hypoglycemia increases due to the additive glucose-lowering effects. Consider reducing basal insulin doses when initiating semaglutide, with subsequent titration based on self-monitored blood glucose values and hemoglobin A1c trends. The American Diabetes Association (ADA) Standards of Care recommends individualized insulin adjustments based on hypoglycemia risk and glycemic targets. Patients should be educated about hypoglycemia recognition and management, including the importance of carrying fast-acting carbohydrates.
Sulfonylureas (such as glipizide, glyburide, and glimepiride) also increase hypoglycemia risk when combined with semaglutide. These medications stimulate insulin secretion independent of glucose concentrations, creating potential for excessive insulin release when used alongside GLP-1 receptor agonists. Consider reducing sulfonylurea doses when starting semaglutide, particularly in patients with baseline A1c values below 8%. Close glucose monitoring during the first 4-8 weeks of combination therapy helps identify patients requiring further dose adjustments.
Metformin, SGLT2 inhibitors, and thiazolidinediones generally do not require dose modification when combined with semaglutide, as hypoglycemia risk remains low with these agents. However, DPP-4 inhibitors (such as sitagliptin or linagliptin) should be discontinued when initiating semaglutide, as both drug classes work through incretin pathways, and combined use offers no additional glycemic benefit while potentially increasing adverse effects. This recommendation aligns with ADA Standards of Care. The choice of combination therapy should be individualized based on patient-specific factors, including baseline glycemic control, hypoglycemia history, renal function, and cardiovascular risk profile.
Patients should proactively communicate with their healthcare providers about all medications, supplements, and over-the-counter products before starting semaglutide. Critical timing for these discussions includes the initial prescribing visit, any dose escalation appointments, and whenever new medications are added to the treatment regimen. Patients should specifically inform their doctor about diabetes medications (insulin, sulfonylureas, meglitinides), thyroid medications, anticoagulants, oral contraceptives, and any medications requiring precise timing or absorption. Those taking oral semaglutide (Rybelsus) should discuss the 30-minute pre-food, pre-medication timing requirement with their healthcare provider.
Warning signs requiring immediate medical attention include symptoms of hypoglycemia (shakiness, sweating, confusion, rapid heartbeat, dizziness), which may indicate that diabetes medication doses need adjustment. Severe hypoglycemia with confusion or unconsciousness requires emergency assistance (call 911). Patients should also contact their healthcare provider if they experience severe or persistent gastrointestinal symptoms (nausea, vomiting, diarrhea) that interfere with taking other essential medications. Severe, persistent abdominal pain (possible pancreatitis), signs of gallbladder disease, or unusual bleeding in patients taking anticoagulants warrant prompt medical evaluation.
Routine monitoring and follow-up should occur at regular intervals, typically every 3-4 months initially, then every 6 months once stable. These visits should include medication reconciliation, review of blood glucose logs (for diabetes patients), assessment of adverse effects, and laboratory monitoring as appropriate (A1c, thyroid function tests, INR for anticoagulated patients). Patients should maintain an updated medication list, including dosages and timing, and bring this to all healthcare appointments. Patients should also report substantial weight loss or dietary changes that could affect medications like warfarin. Pharmacists serve as an additional resource for identifying potential interactions and can provide guidance on optimal medication timing strategies.
FDA studies show no clinically relevant reduction in oral contraceptive efficacy with semaglutide, and no backup contraception is routinely recommended. However, severe vomiting or diarrhea from semaglutide side effects could potentially affect absorption, as with any gastrointestinal illness.
Yes, semaglutide can be safely combined with metformin without dose adjustments, as hypoglycemia risk remains low with this combination. This pairing is commonly used in type 2 diabetes management.
Oral semaglutide (Rybelsus) requires specific timing to ensure proper absorption—it must be taken first thing in the morning with no more than 4 ounces of plain water, at least 30 minutes before food or other medications. This timing requirement does not apply to injectable semaglutide formulations.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.