LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Why do I still feel hungry on semaglutide? This common concern affects many patients using this GLP-1 receptor agonist for diabetes or weight management. While semaglutide effectively reduces appetite in most individuals, persistent hunger can occur due to insufficient dosing duration, hormonal adaptation, dietary factors, or individual variation in medication response. Understanding the reasons behind continued hunger—and knowing when to seek medical guidance—helps optimize treatment outcomes. This article examines the mechanisms of semaglutide's appetite effects, common causes of persistent hunger, and evidence-based strategies to address this challenge during therapy.
Quick Answer: Persistent hunger on semaglutide may occur due to insufficient time at therapeutic dose, hormonal adaptation during weight loss, dietary composition, medication interactions, or individual variation in GLP-1 receptor response.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (under the brand name Ozempic) and chronic weight management (Wegovy). Its mechanism of action involves mimicking the naturally occurring GLP-1 hormone, which plays a central role in glucose homeostasis and appetite regulation.
When administered subcutaneously, semaglutide binds to GLP-1 receptors located throughout the body, including areas that influence appetite control. This activation triggers several physiological responses: it slows gastric emptying (an effect that may attenuate over time with continued use), which initially prolongs the sensation of fullness after meals; it enhances glucose-dependent insulin secretion from pancreatic beta cells; and it reduces glucagon release, thereby lowering hepatic glucose production. Semaglutide affects central appetite pathways primarily through activation of receptors in the area postrema and other circumventricular organs, with additional effects mediated via vagal signaling pathways.
Clinical studies have shown that semaglutide can reduce energy intake in controlled settings, with significant variability between individuals. The medication's long half-life of approximately one week allows for once-weekly dosing and sustained action between injections. However, individual responses vary considerably, and the degree of appetite reduction differs among patients. Understanding this pharmacological foundation helps contextualize why some individuals may experience persistent hunger despite treatment, as the biological response to GLP-1 receptor agonism involves complex interactions between peripheral and central nervous system pathways.
Several physiological and behavioral factors can explain why hunger persists in some patients receiving semaglutide therapy. Insufficient time at therapeutic dose is among the most common reasons. Semaglutide requires gradual dose escalation to minimize gastrointestinal adverse effects, typically starting at 0.25 mg weekly and increasing every four weeks. Many patients do not experience maximal appetite suppression until reaching maintenance doses of 0.5-2.0 mg (Ozempic for diabetes) or up to 2.4 mg (Wegovy for weight management). The full therapeutic effect may take 16-20 weeks to manifest, particularly with the Wegovy titration schedule.
Hormonal and metabolic adaptation represents another significant factor. As patients lose weight, the body undergoes compensatory changes including increased production of ghrelin (the "hunger hormone") and decreased leptin levels. These adaptations can partially counteract semaglutide's appetite-suppressing effects, particularly after several months of treatment. Research indicates that weight loss triggers neurohormonal responses designed to defend against further weight reduction, creating a biological drive to eat that may override medication effects in susceptible individuals.
Dietary composition and meal timing also influence hunger perception during semaglutide treatment. Diets high in refined carbohydrates and low in protein or fiber can cause rapid blood glucose fluctuations, triggering hunger signals despite adequate caloric intake. Additionally, some patients confuse other sensations—such as thirst, fatigue, or emotional distress—with physical hunger. Medication interactions may also play a role; certain medications including corticosteroids, antipsychotics (such as olanzapine, quetiapine), and some antidepressants (particularly mirtazapine) can increase appetite and potentially diminish semaglutide's effects. Finally, individual variation in response to GLP-1 receptor agonist therapy exists, though the specific genetic and physiological factors responsible remain an area of ongoing research.
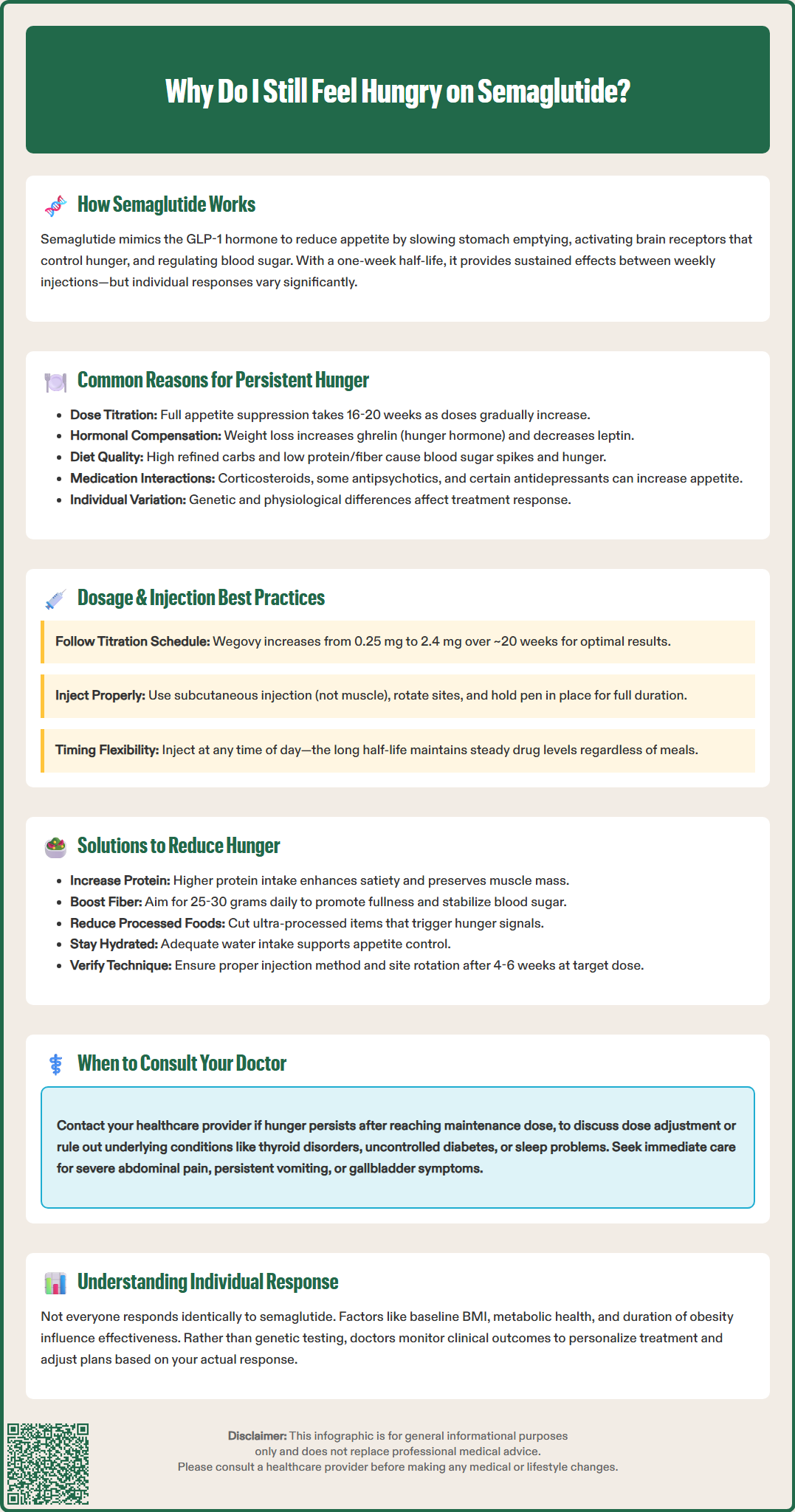
The relationship between semaglutide dosage and appetite suppression follows a dose-response curve, but individual variability significantly affects outcomes. Dose optimization is critical for achieving therapeutic benefit. For type 2 diabetes, the FDA-approved maintenance doses of Ozempic range from 0.5 mg to 2.0 mg weekly, while the weight management indication (Wegovy) follows a specific titration schedule (0.25→0.5→1.0→1.7→2.4 mg at approximately 4-week intervals), with a maximum dose of 2.4 mg weekly. Some patients may maintain at 1.7 mg if the 2.4 mg dose is not tolerated. Clinical evidence from the STEP trials demonstrated that higher doses produce greater weight loss, with the 2.4 mg dose resulting in approximately 15% body weight reduction over 68 weeks compared to placebo.
Due to semaglutide's long half-life and sustained action, the timing of administration relative to meals does not significantly impact its efficacy. Unlike shorter-acting GLP-1 agonists, semaglutide can be injected at any time of day, with or without food, maintaining therapeutic plasma concentrations throughout the week. If a dose is missed, patients should follow the specific instructions in the FDA prescribing information, which differ between Ozempic and Wegovy.
Individual response factors may influence treatment outcomes. Body mass index (BMI) at baseline, metabolic health status, and duration of obesity can all affect response to therapy. Importantly, patients should follow proper injection technique according to the Instructions for Use, including subcutaneous administration (not intramuscular), site rotation between the abdomen, thigh, or upper arm, and holding the pen in place for the recommended time after injection.
While research into predictors of response is ongoing, there is currently insufficient evidence to recommend routine genetic testing to predict individual responses to semaglutide therapy. Healthcare providers should monitor response and adjust treatment plans based on clinical outcomes rather than presumed demographic factors.
When hunger persists despite semaglutide therapy, a systematic approach to evaluation and management is warranted. First, verify adequate dosing and duration. Patients should confirm they have reached their target maintenance dose and have been at that dose for at least 4–6 weeks before concluding that appetite suppression is inadequate. Reviewing injection technique is essential—ensure proper subcutaneous administration with site rotation between the abdomen, thigh, or upper arm, following the specific Instructions for Use for your medication. Missed doses or inconsistent weekly scheduling can significantly diminish therapeutic effects.
Dietary optimization should be addressed through consultation with a registered dietitian. Evidence-based strategies include increasing protein intake to meet individual needs, emphasizing high-fiber foods (aiming for 25–30 g daily per Dietary Guidelines for Americans), and minimizing ultra-processed foods to help stabilize blood glucose and prolong fullness. Adequate hydration is important, with fluid needs varying by individual factors including activity level, climate, and health conditions.
Medical evaluation is indicated if hunger persists despite optimization. Healthcare providers should assess for conditions that increase appetite, including uncontrolled diabetes (check HbA1c), thyroid disorders (TSH, free T4), and psychiatric conditions. Review all concurrent medications for appetite-stimulating effects. Consider screening for sleep disorders, as poor sleep quality can increase appetite.
Dose adjustment may be appropriate in consultation with the prescribing physician. For patients on Ozempic, discussing a higher dose within the approved range may be warranted. Transitioning from Ozempic to Wegovy requires re-titration starting at 0.25 mg and is only appropriate for patients meeting BMI criteria (≥30 kg/m² or ≥27 kg/m² with weight-related comorbidities). Insurance coverage varies significantly.
If hunger remains problematic despite these interventions, referral to an obesity medicine specialist or endocrinologist is appropriate for complex cases. Patients should be counseled that some degree of hunger is normal and does not necessarily indicate treatment failure.
Important safety note: Contact your healthcare provider immediately if you experience severe abdominal pain (possible pancreatitis), symptoms of gallbladder disease, persistent vomiting/inability to keep fluids down, or thoughts of self-harm while taking semaglutide.
Semaglutide typically requires 16–20 weeks to reach maximum appetite suppression, as the medication follows a gradual dose escalation schedule. Full therapeutic effects occur after reaching and maintaining the target maintenance dose for 4–6 weeks.
Yes, dietary composition significantly influences hunger on semaglutide. Diets high in refined carbohydrates and low in protein or fiber can cause blood glucose fluctuations that trigger hunger signals despite adequate caloric intake.
Dose adjustments should only be made in consultation with your prescribing physician. If you have not yet reached the maximum approved dose for your indication and have been at your current dose for at least 4–6 weeks, your healthcare provider may consider increasing within the FDA-approved range.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.