LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Accidentally injected semaglutide into vein is an extremely rare occurrence due to the short needle design of FDA-approved injection pens. Semaglutide (Ozempic, Wegovy) is formulated exclusively for subcutaneous administration, where it absorbs gradually over days. While intravenous injection is highly unlikely with proper technique, understanding what to do if an injection error occurs—and how to prevent it—is essential for safe medication use. This guide covers immediate steps after suspected injection errors, symptoms to monitor, proper injection technique, and when to seek medical care.
Quick Answer: Accidental intravenous injection of semaglutide is extremely unlikely with standard pen devices but would theoretically cause immediate systemic exposure rather than the intended gradual absorption from subcutaneous tissue.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist that is FDA-approved for subcutaneous administration only. The medication is specifically formulated for injection into subcutaneous tissue, where it is absorbed gradually into the systemic circulation. Accidental intravenous injection, while extremely unlikely with the standard pen devices, would theoretically result in immediate systemic exposure rather than the intended slow absorption profile.
There is limited published data on accidental intravenous semaglutide injection in humans, as this route of administration is not studied in clinical trials or included in FDA labeling. The subcutaneous route allows for controlled pharmacokinetics, with peak plasma concentrations occurring 1–3 days after injection and a half-life of approximately one week. Direct venous administration would bypass this absorption phase, potentially altering the medication's pharmacokinetic profile.
The short needle design of semaglutide injection devices (4–8 mm) makes intravenous injection highly unlikely in typical injection sites such as the abdomen, thigh, or upper arm. Ozempic and Wegovy pen devices are designed specifically for subcutaneous administration, with features that do not permit aspiration or reliable visualization of blood return that would confirm intravenous placement.
If you suspect an injection error with semaglutide—such as unusual resistance during injection or injection directly over a visible vein—remain calm and take systematic action. First, withdraw the needle immediately and apply gentle pressure to the injection site with clean gauze or a cotton ball for 1–2 minutes to minimize bleeding and prevent hematoma formation. Do not rub the area, as this may increase local irritation.
Document the incident details including the exact time, injection site location, dose administered (if any), and any immediate symptoms experienced. Take a photograph of the injection site if visible changes such as bruising or swelling develop. Save the pen and note the lot number for potential reporting.
Contact your healthcare provider promptly to report the incident. You can also call U.S. Poison Control at 1-800-222-1222 for immediate guidance on medication errors while awaiting your provider's response. For semaglutide products, the manufacturer maintains medical information services that can provide additional guidance.
Do not attempt to administer a replacement dose unless specifically instructed by a healthcare professional. If you're uncertain how much medication was delivered, seek clinical guidance rather than re-dosing.
Monitor yourself closely for the next 24–48 hours, noting any unusual symptoms. If severe symptoms develop—including difficulty breathing, chest pain, severe nausea or vomiting, or altered consciousness—seek emergency medical care immediately. Consider reporting the incident to FDA MedWatch if adverse effects occur.
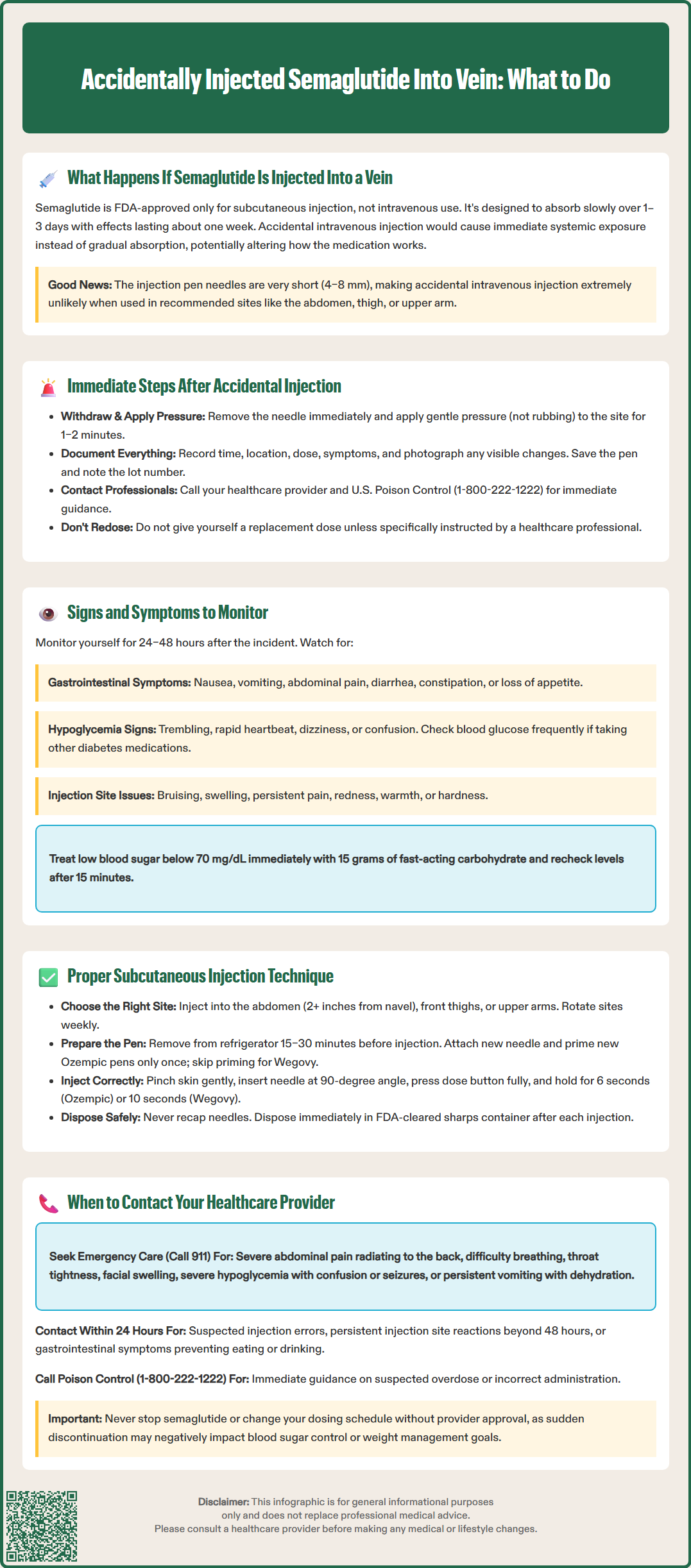
Following a suspected injection error, vigilant monitoring for adverse effects is essential. The most common adverse effects associated with therapeutic semaglutide use include gastrointestinal symptoms, which might theoretically occur more rapidly or intensely with direct venous administration. Monitor for:
Gastrointestinal symptoms:
Nausea or vomiting (occurring in approximately 15–20% of patients at therapeutic doses per FDA labeling)
Abdominal pain or cramping
Diarrhea or constipation
Loss of appetite or early satiety
Bloating or abdominal distension
Hypoglycemia indicators (particularly if you take other diabetes medications):
Trembling, shakiness, or sweating
Rapid heartbeat or palpitations
Dizziness, lightheadedness, or confusion
Hunger or irritability
Headache or blurred vision
Injection site reactions:
Bruising, swelling, or hematoma formation
Persistent pain or tenderness
Redness or warmth suggesting inflammation
Hardness or nodule formation under the skin
Semaglutide's mechanism involves glucose-dependent insulin secretion, meaning hypoglycemia risk is generally low when used as monotherapy. However, if you take sulfonylureas or insulin concurrently, monitor blood glucose more frequently for 24–48 hours after the incident. Check blood glucose immediately if hypoglycemia symptoms develop, and treat with 15 grams of fast-acting carbohydrate if levels fall below 70 mg/dL, rechecking in 15 minutes per American Diabetes Association guidelines.
Rare but serious adverse effects include pancreatitis, gallbladder disease, and dehydration leading to acute kidney injury. Seek immediate medical attention if you experience severe, persistent abdominal pain (especially in the right upper quadrant or radiating to the back), signs of dehydration (reduced urination, extreme thirst), signs of anaphylaxis (difficulty breathing, throat swelling, widespread rash), or any symptom that seems disproportionate to your usual medication response.
Correct subcutaneous injection technique minimizes the risk of injection errors and ensures optimal medication absorption. Semaglutide should be administered into subcutaneous tissue—the fatty layer between skin and muscle—in approved injection sites: the abdomen (at least 2 inches from the navel), front of the thighs, or upper outer arms. Upper arm injections generally require assistance from another person per FDA labeling. Rotate injection sites weekly to prevent lipodystrophy, which can affect absorption and cause cosmetic changes.
For Ozempic (multi-dose pen with detachable needles):
Remove the pen from refrigeration 15–30 minutes before injection to reduce discomfort
Wash hands thoroughly with soap and water
Attach a new needle for each injection
Perform a flow check before first use of each new pen only (not before every dose)
Clean the injection site with an alcohol swab and allow to dry completely
For Wegovy (single-dose pen with built-in needle):
Remove the pen from refrigeration 15–30 minutes before injection
Wash hands thoroughly
Do not prime the pen (it comes ready to use)
Clean the injection site with an alcohol swab and allow to dry completely
Injection technique for both products: Pinch a fold of skin gently between thumb and forefinger to elevate subcutaneous tissue away from underlying muscle. This technique is particularly important for lean individuals. Insert the needle at a 90-degree angle to the skin surface. Depress the dose button fully and maintain pressure while counting slowly to 6 seconds for Ozempic or 10 seconds for Wegovy before withdrawing the needle. This dwell time ensures complete dose delivery. Release the skin pinch before or during needle withdrawal. Do not aspirate (pull back) before injecting, as this is not recommended for subcutaneous injections.
Post-injection care: Dispose of the used needle/pen immediately in an FDA-cleared sharps container. Never recap needles, as this increases needlestick injury risk. If a drop of blood appears at the injection site, apply gentle pressure with clean gauze. Store Ozempic pens (without attached needle) at 36–46°F (2–8°C) or at room temperature up to 86°F (30°C) for up to 56 days after first use. Wegovy pens are single-use and should be discarded after injection.
Certain situations following semaglutide injection warrant prompt healthcare provider contact, even if symptoms seem mild initially. Contact your provider within 24 hours if you suspect any injection error, including possible intravenous administration, intramuscular injection, or incomplete dose delivery. Early notification allows for appropriate monitoring guidance and documentation in your medical record.
Contact your healthcare provider promptly for:
Suspected injection errors or technique concerns
Persistent injection site reactions lasting more than 48 hours
Gastrointestinal symptoms that interfere with eating, drinking, or medication adherence
Blood glucose patterns that differ significantly from your usual readings
Questions about whether to administer a replacement dose after injection error
Call U.S. Poison Control (1-800-222-1222) for:
Immediate guidance on suspected overdose or incorrect administration
Questions about potential medication interactions after an injection error
Seek immediate emergency care (call 911 or go to the emergency department) if you experience:
Severe, persistent abdominal pain, especially if radiating to the back (possible pancreatitis)
Right upper quadrant pain, fever, or yellowing of skin/eyes (possible gallbladder disease)
Signs of severe allergic reaction: difficulty breathing, throat tightness, facial or tongue swelling, widespread rash or hives
Symptoms of severe hypoglycemia: confusion, loss of consciousness, seizures, inability to swallow
Persistent vomiting preventing fluid intake, with signs of dehydration (decreased urination, extreme thirst, dizziness)
Sudden or severe vision changes, particularly if you have diabetic retinopathy
Severe chest pain or rapid, irregular heartbeat
For non-urgent injection technique questions or minor side effects, schedule a routine follow-up appointment or utilize your healthcare system's patient portal messaging. Many providers offer diabetes education services with certified diabetes care and education specialists who can observe your injection technique and provide personalized guidance. Injection technique should be reviewed regularly and with any device changes.
Maintain open communication with your healthcare team about any concerns regarding semaglutide administration. Never discontinue semaglutide or alter your dosing schedule without provider guidance, as abrupt cessation may affect glycemic control or weight management goals depending on your indication for use.
No, semaglutide is FDA-approved only for subcutaneous injection and is not formulated or studied for intravenous use. The short needle design of Ozempic and Wegovy pens makes accidental intravenous injection extremely unlikely when proper technique is used.
Withdraw the needle immediately, apply gentle pressure to the injection site for 1–2 minutes, document the incident details, and contact your healthcare provider promptly. Do not administer a replacement dose without medical guidance.
Use proper subcutaneous technique by pinching skin, inserting the needle at a 90-degree angle in approved sites (abdomen, thigh, or upper arm), and maintaining pressure on the dose button for the full dwell time (6–10 seconds). Rotate injection sites weekly and never inject directly over visible veins.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.