LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Does GLP-1 help with lipedema? This question reflects growing interest in glucagon-like peptide-1 (GLP-1) receptor agonists for managing this challenging adipose tissue disorder. Lipedema causes symmetrical, disproportionate fat accumulation primarily in the legs and arms, predominantly affecting women. Unlike typical obesity, lipedema fat resists conventional diet and exercise, leaving patients frustrated after years of ineffective treatments. While GLP-1 medications like semaglutide and tirzepatide have demonstrated significant weight loss effects, their specific role in lipedema management remains under investigation. Current evidence consists mainly of case reports rather than controlled trials, and no GLP-1 medication has FDA approval for lipedema treatment.
Quick Answer: GLP-1 receptor agonists are not FDA-approved for lipedema and lack high-quality clinical trial evidence, though preliminary case reports suggest potential symptomatic benefits.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Lipedema is a chronic adipose tissue disorder characterized by symmetrical, disproportionate accumulation of subcutaneous fat, primarily affecting the lower extremities and sometimes the arms. This condition predominantly affects women, with onset typically occurring during hormonal changes such as puberty, pregnancy, or menopause. Unlike obesity, lipedema fat is resistant to diet and exercise, creating significant frustration for patients who often experience years of misdiagnosis.
The pathophysiology of lipedema remains incompletely understood, though current evidence suggests involvement of hormonal factors, genetic predisposition, lymphatic dysfunction, and chronic inflammation. Patients commonly present with pain, tenderness, easy bruising, and progressive swelling that spares the feet, creating a characteristic "cuff" appearance at the ankles. Diagnostic features include symmetrical presentation, negative Stemmer's sign (ability to pinch skin at the base of the toes), and absence of pitting edema.
Current treatment approaches focus on conservative management rather than cure. Standard interventions include complete decongestive therapy (CDT), compression garments, manual lymphatic drainage, and lifestyle modifications. However, these treatments primarily address symptoms and lymphatic complications rather than the underlying adipose tissue abnormality. Liposuction, specifically tumescent technique or water-assisted liposuction, represents the only treatment that directly removes lipedema fat, but it is invasive, costly, often requires multiple staged procedures, and is not universally accessible.
The resistance of lipedema tissue to conventional weight loss methods has prompted investigation into pharmacological approaches. Given the metabolic and inflammatory components of lipedema, medications that address these pathways have generated clinical interest, including newer incretin-based therapies originally developed for type 2 diabetes and obesity management.
Importantly, patients experiencing sudden unilateral swelling, erythema with fever, or severe calf pain should seek immediate medical attention to rule out conditions requiring urgent intervention such as deep vein thrombosis, cellulitis, or lymphedema.
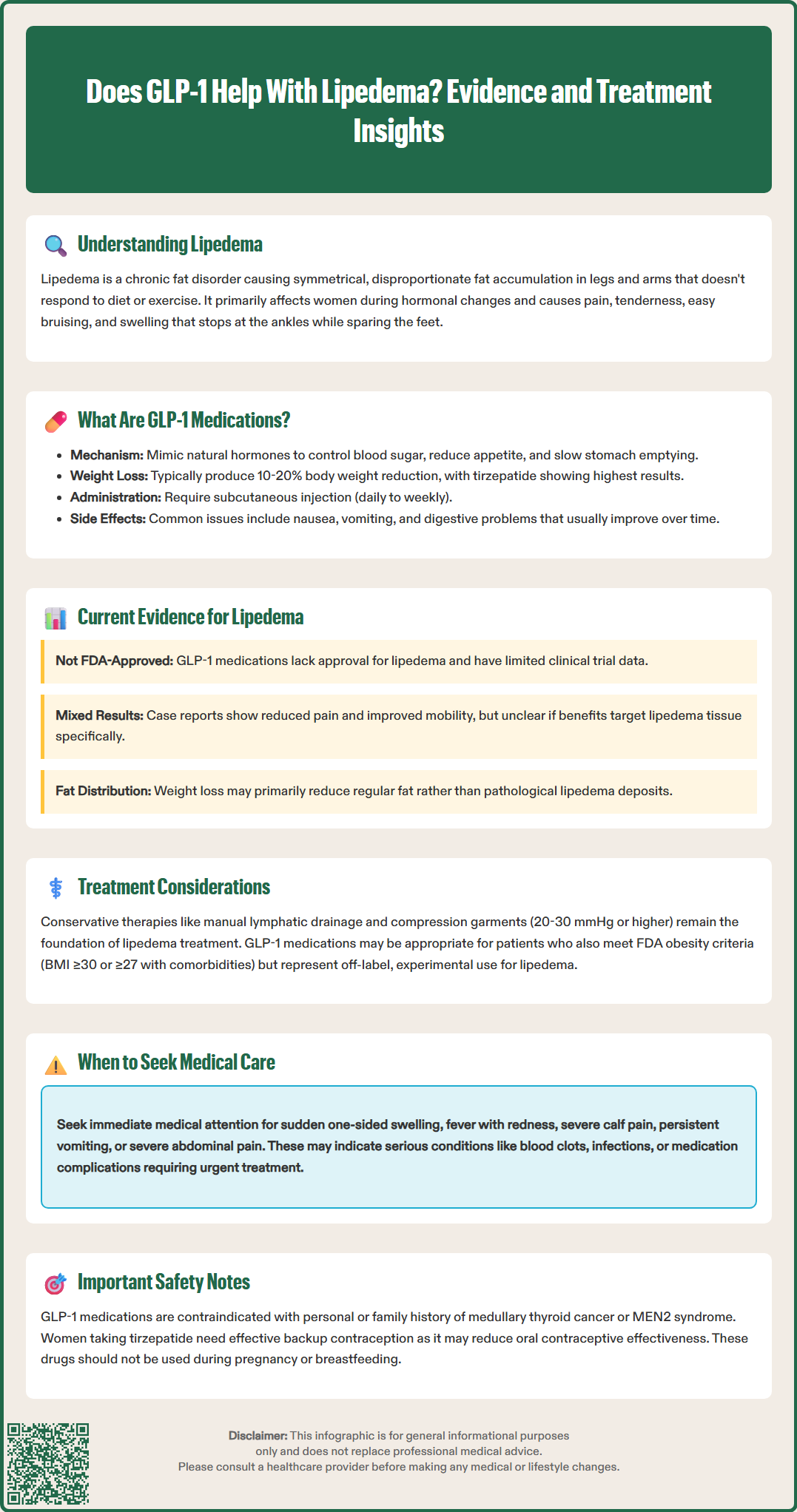
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications that mimic the action of the naturally occurring incretin hormone GLP-1. This class includes semaglutide (Ozempic for diabetes, Wegovy for weight management) and liraglutide (Victoza for diabetes, Saxenda for weight management). Tirzepatide (Mounjaro for diabetes, Zepbound for weight management) is a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, not a pure GLP-1 agonist. Dulaglutide (Trulicity) is FDA-approved only for type 2 diabetes management, not for weight loss.
The mechanism of action involves binding to GLP-1 receptors located throughout the body, including the pancreas, brain, gastrointestinal tract, and cardiovascular system. In the pancreas, these medications enhance glucose-dependent insulin secretion and suppress inappropriate glucagon release, improving glycemic control. In the central nervous system, they act on appetite-regulating centers in the hypothalamus, reducing hunger and increasing satiety signals. Additionally, they slow gastric emptying, prolonging the feeling of fullness after meals.
Some research suggests these medications may have anti-inflammatory properties and could influence adipose tissue metabolism, though these effects vary by agent and require further study. Clinical trials have shown that weight loss with these medications typically ranges from 10-20% of body weight, depending on the specific agent and dosage, with tirzepatide showing the highest average weight reduction.
Common adverse effects include gastrointestinal symptoms (nausea, vomiting, diarrhea, constipation), which are usually transient and dose-dependent. More serious but rare risks include pancreatitis and gallbladder disease. These medications carry a boxed warning about thyroid C-cell tumors in rodents, and are contraindicated in patients with personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN2). Additional safety considerations include risk of dehydration and acute kidney injury, potential worsening of diabetic retinopathy with rapid glucose improvement (particularly with semaglutide), and hypoglycemia risk when combined with insulin or sulfonylureas.
These medications are not recommended during pregnancy or breastfeeding and require effective contraception in women of childbearing potential. Notably, tirzepatide may decrease the effectiveness of oral contraceptives, necessitating alternative contraceptive methods. All formulations require subcutaneous injection, with dosing frequencies ranging from daily to weekly depending on the specific medication.
Currently, there is no FDA-approved indication for GLP-1 receptor agonists or GIP/GLP-1 dual agonists specifically for lipedema treatment, and high-quality clinical trial evidence remains limited. The existing literature consists primarily of case reports, small case series, and observational studies, which provide preliminary insights but cannot establish definitive efficacy or safety profiles for this specific population.
Several case reports have documented individual patients with lipedema who experienced symptomatic improvement and some reduction in limb circumference while taking incretin-based medications, particularly semaglutide. These reports suggest potential benefits including reduced pain, improved mobility, and decreased inflammation. However, it remains unclear whether observed improvements result from general weight loss, potential anti-inflammatory effects, or direct action on lipedema tissue. Importantly, lipedema fat has historically been considered resistant to weight loss interventions, raising questions about whether GLP-1-induced weight reduction affects lipedema tissue differently than conventional caloric restriction.
A critical limitation in interpreting available evidence is the difficulty distinguishing lipedema fat from concurrent obesity. Many patients with lipedema also have generalized obesity, and weight loss from GLP-1 therapy may primarily reduce non-lipedema adipose tissue while having minimal effect on the pathological lipedema deposits. Some clinicians report that patients experience proportional fat loss, meaning the characteristic disproportion between affected and unaffected areas persists despite overall weight reduction.
The hypothesized anti-inflammatory properties of these medications represent a theoretically attractive mechanism for lipedema management, given the inflammatory component of the condition. However, no controlled studies have specifically examined inflammatory markers in lipedema patients treated with these medications. Additionally, questions exist about potential effects in this population, including the impact of rapid weight loss on skin elasticity and lymphatic function in areas already affected by tissue changes.
For meaningful assessment of treatment response, standardized measurements should be employed, including limb circumference at defined anatomical points, validated pain scales, and quality-of-life instruments specific to lipedema or lymphatic conditions.
Given the current evidence gaps, incretin-based medications should not be considered established lipedema treatments. Patients considering these medications should understand they are being used off-label for this indication, and expectations should be carefully managed regarding potential benefits and limitations.
Comprehensive lipedema management requires a multimodal approach tailored to individual patient needs, disease stage, and concurrent conditions. Conservative therapies remain the foundation of treatment and should be optimized before or alongside any pharmacological interventions. Complete decongestive therapy, including manual lymphatic drainage performed by trained therapists, helps manage fluid accumulation and may reduce discomfort. Compression garments (20-30 mmHg or higher) provide mechanical support and help control swelling, though patient adherence can be challenging due to discomfort and difficulty with application.
Lifestyle modifications, while not curative for lipedema tissue itself, support overall health and may help manage concurrent obesity. A balanced diet following evidence-based patterns such as the Mediterranean diet may support metabolic health and potentially reduce inflammation. Low-impact exercise such as swimming, water aerobics, and walking can improve mobility and lymphatic flow without exacerbating pain. Patients should be counseled that traditional weight loss approaches will not eliminate lipedema fat but can improve overall metabolic health.
For patients considering GLP-1 receptor agonists or GIP/GLP-1 dual agonists, several factors warrant careful evaluation. These agents may be appropriate for individuals with lipedema who also meet FDA criteria for obesity treatment (BMI ≥30 kg/m² or ≥27 kg/m² with weight-related comorbidities) or have concurrent type 2 diabetes. A thorough assessment should include screening for contraindications such as personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Caution is advised in patients with a history of pancreatitis or severe gastrointestinal disease including gastroparesis.
These medications are not recommended during pregnancy or breastfeeding, and effective contraception should be used during treatment. Women taking tirzepatide should be advised that it may decrease the effectiveness of oral contraceptives. Monitoring should include assessment of gastrointestinal tolerance, hydration status, renal function, limb measurements, pain levels, mobility, and quality of life indicators. Patients with diabetes require additional monitoring for retinopathy and hypoglycemia, especially if taking insulin or sulfonylureas.
Patients should be informed that incretin therapy for lipedema represents off-label use with uncertain benefits specific to lipedema tissue. Realistic expectations should emphasize that these medications may help with overall weight management and potentially reduce inflammation and pain, but are unlikely to eliminate the characteristic fat distribution of lipedema. Insurance coverage for off-label use may be limited, creating significant financial barriers for many patients.
Referral to specialists experienced in lipedema management is advisable for diagnostic confirmation and treatment planning. Patients should seek immediate medical attention for signs of possible complications such as cellulitis (redness, warmth, fever), deep vein thrombosis (sudden unilateral swelling, calf pain), or significant worsening of symptoms. Any new or worsening symptoms while on therapy, including severe abdominal pain, persistent nausea or vomiting, or signs of gallbladder disease, require prompt medical evaluation.
No, GLP-1 receptor agonists are not FDA-approved for lipedema treatment. They are approved for type 2 diabetes management and obesity treatment in patients meeting specific BMI criteria, but their use for lipedema represents off-label prescribing with limited clinical evidence.
GLP-1 medications are unlikely to eliminate characteristic lipedema fat distribution. While they may help with overall weight management and potentially reduce inflammation and pain, lipedema fat has historically been resistant to weight loss interventions, and the characteristic disproportion between affected and unaffected areas typically persists.
GLP-1 medications are contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. They are not recommended during pregnancy or breastfeeding, and caution is advised in patients with history of pancreatitis or severe gastrointestinal disease.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.