LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

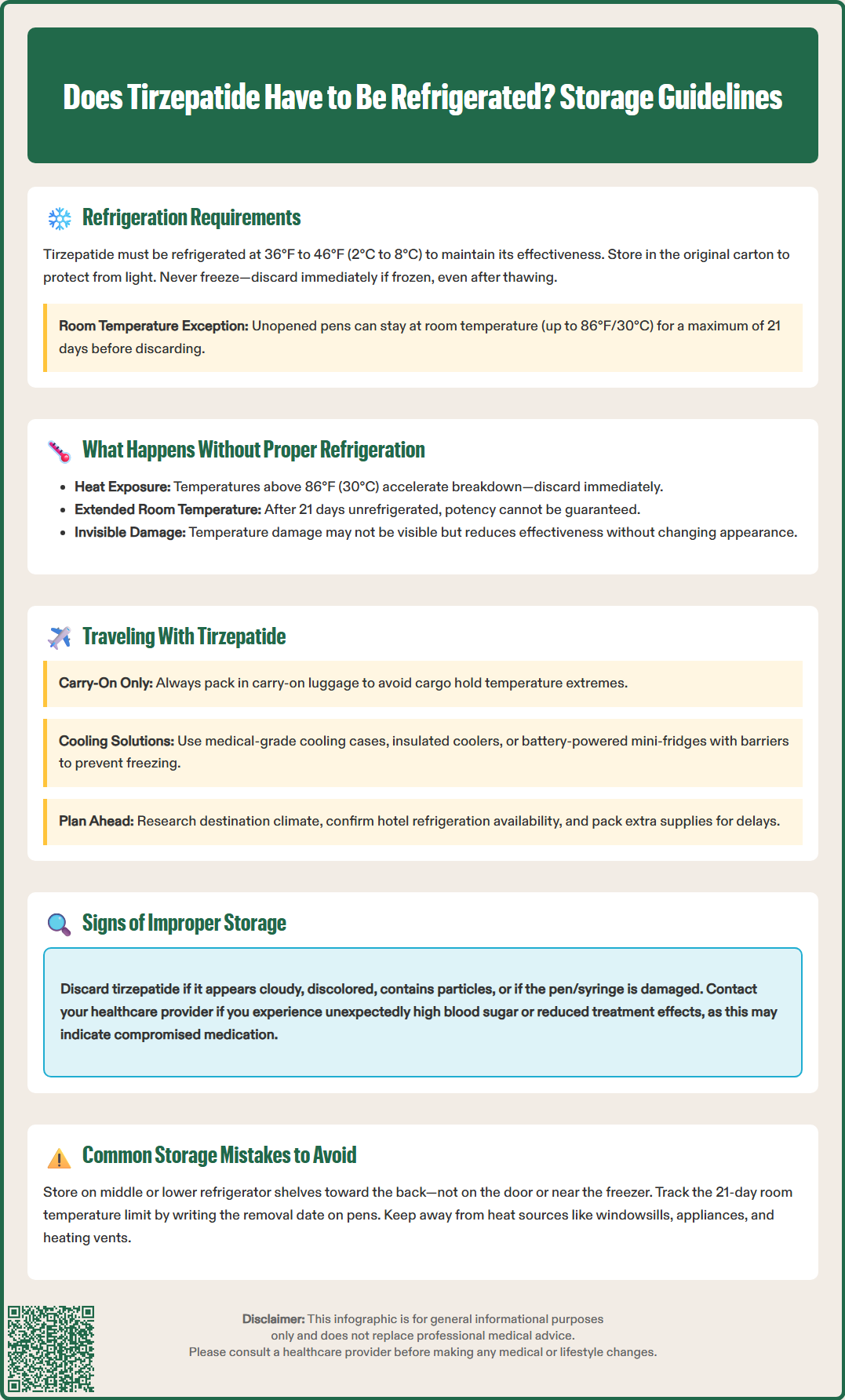
Does tirzepatide have to be refrigerated? Yes, tirzepatide must be stored in a refrigerator at 36°F to 46°F (2°C to 8°C) to maintain its stability and effectiveness. This dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist is a large peptide molecule that degrades when exposed to improper temperatures. Both Mounjaro and Zepbound (tirzepatide brands) require refrigeration according to FDA-approved labeling. Understanding proper storage requirements ensures your medication remains therapeutically effective and safe for administration. This guide covers essential storage guidelines, travel considerations, and how to identify improperly stored medication.
Quick Answer: Tirzepatide must be refrigerated at 36°F to 46°F (2°C to 8°C) to maintain stability and therapeutic effectiveness.
Yes, tirzepatide must be refrigerated to maintain its stability and therapeutic effectiveness. According to the FDA-approved prescribing information for both Mounjaro and Zepbound (tirzepatide), the medication should be stored in a refrigerator at temperatures between 36°F to 46°F (2°C to 8°C). This temperature range is critical for preserving the peptide structure of this glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist.
Tirzepatide is a large peptide molecule that can degrade when exposed to temperatures outside the recommended range. The medication should be stored in its original carton to protect it from light until you are ready to use it. Never freeze tirzepatide—if the medication has been frozen, it must be discarded, as the FDA labeling instructs not to use the product if it has been frozen.
If needed, unopened tirzepatide pens or syringes may be kept at room temperature (up to 86°F or 30°C) for a maximum of 21 days before use. After this period, the medication should be discarded. It's important to understand that tirzepatide devices (Mounjaro pens/syringes and Zepbound pens) are single-dose—they should be used once and then discarded immediately after injection, regardless of the storage method.
When receiving tirzepatide, especially if shipped, check that it arrives with appropriate cooling elements and has not been exposed to extreme temperatures during transit. Refrigerate the medication promptly upon receipt. Your healthcare provider should counsel you on proper storage during the initial prescription visit.
When tirzepatide is stored outside recommended temperature parameters, the medication's chemical stability becomes compromised. Peptide degradation occurs when temperature and time exceed guidelines. This degradation doesn't necessarily make the medication visibly different, but it can reduce therapeutic potency.
If an unopened tirzepatide pen or syringe has been kept at room temperature (below 86°F/30°C) for less than 21 days, the medication remains safe and effective to use. However, once this 21-day window expires, the medication should be discarded as the manufacturer cannot guarantee its potency or sterility.
Exposure to temperatures above 86°F (30°C) accelerates degradation. If tirzepatide has been exposed to high heat—such as being left in a hot car, near a window with direct sunlight, or next to heat sources—it should be discarded according to FDA guidelines.
Freezing presents a different concern: the medication should not be used if it has been frozen. A frozen pen or syringe must be discarded immediately, even if it has thawed. If you're unsure about your medication's storage history, contact your pharmacist or healthcare provider before administering the dose—patient safety should always take precedence over medication cost concerns.
If you notice unexpectedly elevated blood glucose readings or diminished therapeutic effects, discuss these changes with your healthcare provider. While many factors can affect glycemic control and weight management, changes in medication effectiveness could potentially be related to storage issues.
Traveling with tirzepatide requires advance planning to maintain proper storage conditions throughout your journey. For short trips (under 21 days), unopened tirzepatide pens or syringes can be transported at room temperature without additional cooling, provided ambient temperatures remain below 86°F (30°C). Keep the medication in your carry-on luggage rather than checked baggage, as cargo holds can experience temperature extremes and freezing conditions at high altitudes.
For extended travel or trips to warm climates, consider using a medical-grade cooling case specifically designed for injectable medications. These portable solutions include:
Insulated medication coolers with reusable ice packs
Battery-powered mini-refrigerators designed for medications
Evaporative cooling wallets that use water evaporation to keep medications cool without electricity
Phase-change cooling pouches that maintain specific temperature ranges
Important: When using cooling devices with ice packs, place a barrier (such as a cloth or bubble wrap) between the medication and the ice pack to prevent freezing the medication through direct contact.
When flying, the Transportation Security Administration (TSA) permits medications and associated cooling accessories in carry-on baggage. Declare these items at screening and keep medications in their original labeled containers. Bring your prescription label or a letter from your healthcare provider confirming your need for the medication.
International travel requires additional considerations. Research your destination's climate and accommodation refrigeration availability. Many hotels may store medication in their refrigerators upon request—call ahead to inquire about this service. For destinations with unreliable electricity, consider locations with backup generators or bring multiple cooling solutions. Always pack extra supplies in case of travel delays, and know how to access medical care and pharmacy services at your destination if replacement medication becomes necessary.
Visual inspection provides the first line of defense in identifying compromised tirzepatide. According to the Instructions for Use, properly stored tirzepatide should appear as a clear, colorless to slightly yellow solution without visible particles. Before injection, hold the pen or syringe up to light and examine the solution carefully. Any of the following signs indicate the medication should not be used:
Cloudiness or turbidity in the solution
Visible particles, flakes, or crystals floating in the liquid
Color changes beyond the normal slight yellow tint (brown, dark yellow, or any other discoloration)
Cracks or damage to the pen or syringe
Unfortunately, peptide degradation from improper temperature storage often occurs without visible changes. This makes tracking storage conditions essential. If you know or suspect your tirzepatide has been exposed to temperatures outside the 36-86°F range, or if an unopened pen/syringe has been at room temperature for more than 21 days, the medication should be discarded regardless of appearance.
If you experience unexpected changes in your response to the medication, such as different blood glucose patterns or changes in appetite control, discuss these observations with your healthcare provider. While such changes could have many causes, they might potentially indicate an issue with medication potency.
When in doubt, do not use the medication. Contact your pharmacist or healthcare provider for guidance. Most pharmacies can verify whether a replacement is warranted and work with your insurance for coverage. Document any storage incidents, including dates, estimated temperatures, and duration of exposure—this information helps your healthcare team make informed decisions and may be necessary for insurance claims or manufacturer replacement programs.
Freezer placement errors represent a common and serious storage mistake. Many patients store tirzepatide on refrigerator door shelves or too close to the freezer compartment, where temperature fluctuations are greatest. The door experiences the most temperature variation with frequent opening and closing, while areas near the freezer can drop below 36°F. Solution: Store tirzepatide in the main refrigerator compartment, ideally in the middle or lower shelves toward the back, where temperature remains most stable. Consider using a refrigerator thermometer to verify your storage area maintains 36-46°F consistently.
Forgetting the 21-day room temperature limit occurs frequently when patients remove unopened pens or syringes from refrigeration. Without tracking, it's easy to exceed the safe room-temperature storage period. Solution: If you remove an unopened pen or syringe from the refrigerator, write the date on the carton. Remember that once you use the single-dose device, it should be discarded immediately after injection.
Improper travel preparation leads to medication exposure during transit. Patients may forget their medication requires refrigeration during travel or may not verify that hotel refrigerators maintain appropriate temperatures. Solution: Plan cooling strategies before departure, test your cooling case at home, and always have a backup plan. Request refrigerator access when booking accommodations.
Storing near heat sources happens when patients don't recognize subtle heat exposure risks. Windowsills with direct sunlight, areas above appliances, or spots near heating vents can all create localized warm zones. Solution: Choose storage locations away from windows, heat sources, and direct sunlight. If storing unopened pens/syringes at room temperature, select the coolest area of your home, typically an interior closet or drawer away from external walls.
Failing to protect from light while less critical than temperature control, can contribute to degradation over time. Solution: Keep tirzepatide in its original carton until use, which provides light protection and helps you track important information like expiration dates and storage start dates. These simple preventive measures help ensure you receive the full therapeutic benefit of your tirzepatide treatment.
Yes, if the unopened tirzepatide pen or syringe was kept at room temperature below 86°F (30°C) for less than 21 days total, it remains safe and effective to use. Track the date you removed it from refrigeration to ensure you stay within the 21-day window.
Discard any tirzepatide that has been frozen, even if it has thawed. According to FDA labeling, frozen medication should not be used as freezing compromises the peptide structure and medication safety.
Keep tirzepatide in your carry-on luggage in its original labeled container. For trips under 21 days, unopened pens can travel at room temperature if conditions stay below 86°F. For longer trips or warm destinations, use a medical-grade cooling case with appropriate barriers to prevent freezing from direct ice pack contact.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.