LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

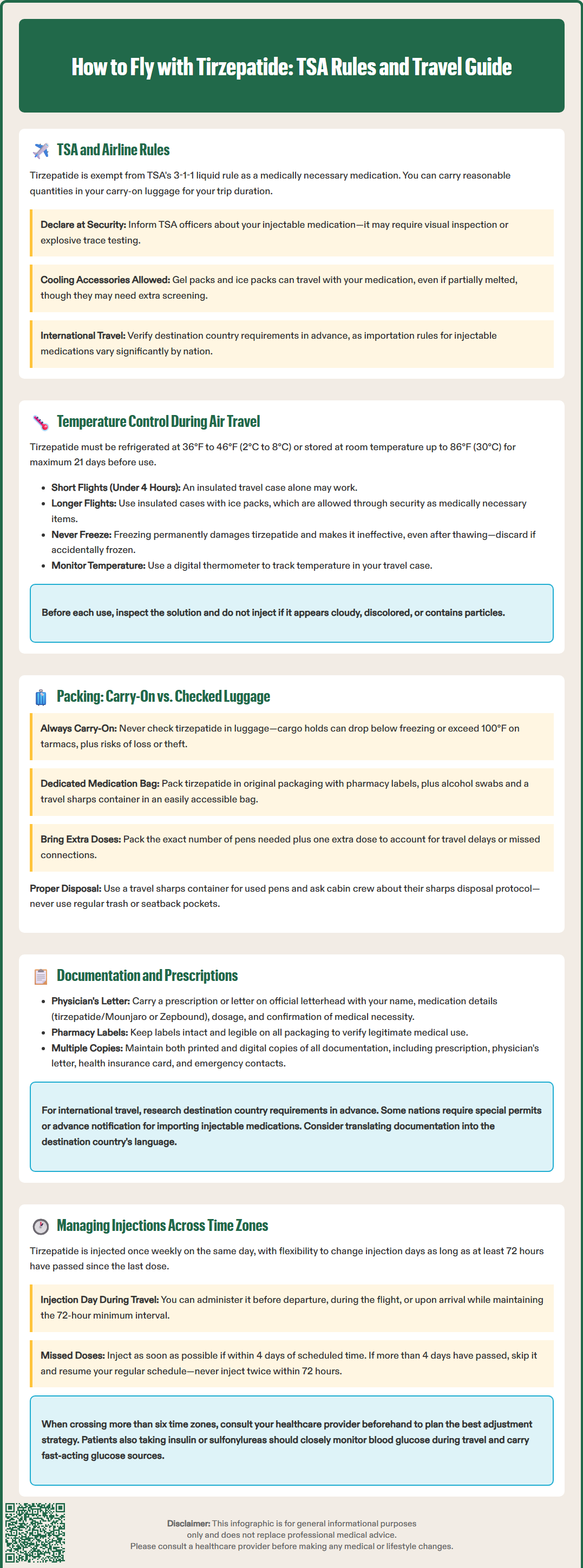
How to fly with tirzepatide requires understanding TSA regulations, proper temperature control, and documentation requirements to ensure safe medication transport. Tirzepatide (Mounjaro, Zepbound) is a once-weekly injectable medication approved by the FDA for type 2 diabetes and chronic weight management. As a temperature-sensitive prescription medication, tirzepatide demands careful planning when traveling by air. This guide covers TSA screening procedures, storage requirements during flights, packing strategies, necessary documentation, and managing your injection schedule across time zones to help you travel confidently with this essential medication.
Quick Answer: Tirzepatide must be carried in cabin baggage with TSA declaration, maintained between 36°F-46°F or at room temperature up to 86°F for maximum 21 days, and accompanied by prescription documentation.
Tirzepatide (Mounjaro, Zepbound) is considered a medically necessary liquid medication by the Transportation Security Administration (TSA), exempting it from the standard 3-1-1 liquid restriction for carry-on items. Passengers may transport tirzepatide pens in reasonable quantities needed for their trip duration, provided they declare these items at the security checkpoint.
TSA officers may conduct additional screening of injectable medications, including visual inspection or testing for trace explosives. When going through security, inform officers that you are traveling with injectable medication. While the TSA does not require prescriptions at security checkpoints, carrying documentation is advisable for reasons discussed below. Cooling accessories such as gel packs or ice packs are permitted when accompanying medications, even if partially melted, though they may require additional screening.
Most domestic and international airlines permit passengers to carry injectable medications and associated supplies, including needles and alcohol swabs, in cabin baggage. However, regulations vary by country and airline. Passengers traveling internationally should verify destination country requirements well in advance, as some nations have strict importation rules for injectable medications. Consider using the TSA Cares helpline (855-787-2227) or carrying a TSA Notification Card to facilitate screening. The TSA recommends arriving at the airport with extra time when traveling with medications to accommodate additional screening procedures. Passengers should never pack tirzepatide in checked luggage due to temperature control concerns and the risk of loss or damage to essential medication.
Tirzepatide requires refrigeration between 36°F and 46°F (2°C to 8°C) until use. According to FDA labeling, tirzepatide pens may be stored at room temperature up to 86°F (30°C) for up to 21 days before use. Maintaining appropriate temperature during air travel is essential to preserve medication efficacy and prevent degradation of the active ingredient.
For flights under four hours, an insulated medication travel case without additional cooling may suffice if the total time out of refrigeration remains within the 21-day limit. For longer flights or when temperature control is a concern, travelers should use an insulated medication travel case with gel packs or ice packs. These cooling accessories are permitted through security as medically necessary items when accompanying medications, even if partially melted or slushy, though they will be subject to additional screening.
Cargo holds of commercial aircraft can experience temperature extremes, with some areas dropping below freezing. Tirzepatide must never be frozen, as freezing permanently damages the medication and renders it ineffective. If tirzepatide accidentally freezes, it must be discarded even if it later thaws. Similarly, exposure to temperatures exceeding 86°F (30°C) may compromise drug stability. Passengers should monitor temperature using small digital thermometers designed for medication travel cases and should never leave tirzepatide in direct sunlight or near heat sources during travel.
Remember that tirzepatide pens are single-dose and should be kept in their original carton to protect from light. Before administration, visually inspect the solution – do not use if the liquid appears cloudy, discolored, or contains particles.
The FDA and American Diabetes Association (ADA) strongly recommend carrying tirzepatide in cabin baggage rather than checked luggage. This recommendation stems from multiple safety and practical considerations that protect both medication integrity and patient access to essential treatment.
Checked luggage compartments experience uncontrolled temperature fluctuations that frequently fall outside tirzepatide's safe storage range. Cargo holds may reach freezing temperatures at cruising altitude or exceed 100°F (38°C) when sitting on tarmacs in warm climates. Additionally, checked bags face risks of loss, theft, or delayed arrival at the destination, potentially leaving patients without necessary medication for extended periods.
When packing tirzepatide in carry-on luggage, use a dedicated medication bag that is easily accessible for security screening. Include all supplies needed for administration: alcohol swabs, a travel sharps container, and any backup supplies. Since tirzepatide pens are single-use, pack the exact number of pens needed for your trip duration plus at least one extra dose to account for travel delays or missed connections. Keep tirzepatide in its original packaging with pharmacy labels clearly visible, which helps identify the medication during security screening and provides dosing information if needed.
For proper disposal of used pens during flights, bring a small travel sharps container and ask cabin crew about their sharps disposal protocol. Never dispose of used pens in regular trash or seatback pockets. For extended international travel requiring multiple weeks of medication, patients should verify that carrying larger quantities is permissible at their destination. Some countries limit the amount of imported medication to a 30- or 90-day supply. Travelers should contact the embassy or consulate of their destination country to confirm specific requirements and obtain necessary permits if required.
While TSA does not mandate prescriptions for domestic flights, carrying comprehensive documentation for tirzepatide provides important protections and facilitates smoother travel, particularly for international destinations. A current prescription or letter from the prescribing physician should include the patient's name, medication name (both brand and generic: tirzepatide/Mounjaro or tirzepatide/Zepbound), dosage, administration route, and confirmation of medical necessity.
The physician's letter should be printed on official letterhead and include contact information for verification purposes. For tirzepatide specifically, the letter might note its indication for type 2 diabetes management (Mounjaro) or chronic weight management (Zepbound), depending on the prescribed formulation. This documentation becomes particularly important when traveling to countries with strict medication importation laws or when carrying quantities that might appear excessive for personal use.
International travelers should research destination country requirements well in advance. Some nations require advance notification or special permits for importing injectable medications, even for personal use. The U.S. Department of State and destination country embassy websites provide specific guidance. Patients should carry documentation in English and, when possible, translated into the destination country's official language. When returning to the U.S., be prepared to declare medications to U.S. Customs and Border Protection (CBP) and show prescriptions if requested.
Pharmacy labels on tirzepatide packaging should remain intact and legible, displaying the patient's name, prescribing physician, medication name, and dispensing pharmacy. These labels serve as additional verification of legitimate medical use. Travelers should also carry their health insurance card and a list of emergency contacts, including their prescribing physician's office number. Keep both printed and digital copies of all documentation. For extended international travel, patients might request a letter confirming their treatment plan and the need to carry multiple doses, which can address questions from customs officials about medication quantities.
Tirzepatide is administered once weekly, providing flexibility that simplifies time zone management compared to daily medications. However, patients should plan their injection schedule thoughtfully when traveling across multiple time zones to maintain consistent dosing intervals.
The FDA-approved dosing schedule for tirzepatide allows administration on the same day each week, with flexibility to adjust the injection day if needed, provided at least 72 hours (three days) have elapsed since the last dose. For flights crossing time zones, patients have several management options. If the scheduled injection day falls during travel, patients may administer the dose before departure, during the flight if comfortable doing so, or upon arrival at the destination, maintaining the minimum 72-hour interval from the previous dose.
If a dose is missed, the FDA labeling instructs patients to administer the missed dose as soon as possible if within 4 days (96 hours) of the scheduled time. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. Never administer two doses within 72 hours of each other.
For significant time zone changes (more than six hours), patients should consult their healthcare provider before travel to determine the optimal adjustment strategy. One approach involves gradually shifting the injection day to align with the destination time zone. For example, a patient who typically injects on Monday mornings in New York and travels to London (five hours ahead) might inject on Monday morning before departure and continue Monday injections in London, effectively maintaining the same weekly schedule despite the time difference.
Patients taking tirzepatide with insulin or sulfonylureas should be particularly vigilant about monitoring blood glucose during travel, as the risk of hypoglycemia may increase with disrupted eating and activity patterns. Always carry fast-acting glucose sources and maintain a medication diary or use smartphone reminders to track injection timing during travel when normal routines are disrupted.
Yes, tirzepatide is exempt from TSA's 3-1-1 liquid restrictions as medically necessary medication. You must declare it at the security checkpoint, and TSA officers may conduct additional screening including visual inspection.
Always pack tirzepatide in carry-on luggage, never in checked bags. Cargo holds experience temperature extremes that can freeze or overheat the medication, and checked luggage risks loss or delayed arrival.
TSA does not require prescriptions for domestic flights, but carrying a current prescription or physician's letter is strongly recommended, especially for international travel where customs regulations may require documentation of medical necessity.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.