LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Tirzepatide is an FDA-approved medication for type 2 diabetes and chronic weight management, available exclusively as pre-filled injection pens under the brand names Mounjaro and Zepbound. Understanding how to properly use tirzepatide is essential for safety and effectiveness. This dual GIP/GLP-1 receptor agonist is administered as a once-weekly subcutaneous injection and must be obtained through legitimate healthcare channels. Importantly, tirzepatide powder forms are not FDA-approved for patient use, and reconstituting or using compounded versions carries significant health risks. This guide provides comprehensive information on proper tirzepatide administration, storage, safety considerations, and when to seek medical guidance.
Quick Answer: FDA-approved tirzepatide is available only as pre-filled injection pens (Mounjaro, Zepbound), not as powder for reconstitution, and should be administered as a once-weekly subcutaneous injection following proper technique and storage guidelines.
Tirzepatide is a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for the treatment of type 2 diabetes mellitus under the brand name Mounjaro and for chronic weight management under the brand name Zepbound. This dual-action medication works by targeting two incretin hormone receptors.
The mechanism of action involves simultaneous activation of both GIP and GLP-1 receptors, which are naturally occurring incretin hormones. When tirzepatide binds to these receptors, it enhances glucose-dependent insulin secretion from pancreatic beta cells, suppresses inappropriately elevated glucagon secretion, and slows gastric emptying. These combined effects result in improved glycemic control in patients with type 2 diabetes. Additionally, tirzepatide acts on appetite regulation centers in the brain, promoting satiety and reducing caloric intake, which contributes to weight loss.
The medication is administered as a once-weekly subcutaneous injection, with dosing typically initiated at 2.5 mg once weekly for 4 weeks, then increased by 2.5 mg every 4 weeks as needed and tolerated up to 15 mg. Maximum approved doses are 15 mg weekly for both diabetes management and weight management. The extended half-life of approximately 5 days allows for convenient weekly dosing, improving adherence compared to daily injectable therapies.
It is important to understand that FDA-approved tirzepatide is supplied as a pre-filled, single-dose pen containing a sterile solution ready for injection. The medication is not approved or intended to be sold, distributed, or reconstituted as a powder for patient use outside of FDA-regulated manufacturing processes. Tirzepatide is not indicated for type 1 diabetes or the treatment of diabetic ketoacidosis.
The only FDA-approved forms of tirzepatide are the pre-filled, single-dose injection pens marketed as Mounjaro (for type 2 diabetes) and Zepbound (for chronic weight management). These devices are manufactured by Eli Lilly and Company under strict quality control standards that ensure sterility, accurate dosing, and product stability. Each pen is designed for single use and contains a precise dose of tirzepatide in a ready-to-inject solution.
Legitimate tirzepatide must be obtained through a valid prescription from a licensed healthcare provider and dispensed by a licensed pharmacy. The medication should arrive in its original packaging with intact seals, proper labeling including lot numbers and expiration dates, and manufacturer information. Patients should verify that their pharmacy is licensed by checking with their state board of pharmacy. For online pharmacies, verify legitimacy through the National Association of Boards of Pharmacy (NABP) Verified Internet Pharmacy Practice Sites (VIPPS) program. Be cautious of unusually low prices or offers that seem too good to be true.
While compounded drugs are not FDA-approved, certain compounding may be permitted under sections 503A and 503B of the Federal Food, Drug, and Cosmetic Act in limited circumstances (such as drug shortages). However, compounded tirzepatide remains unapproved and has not been established as equivalent to FDA-approved products in terms of safety, efficacy, or quality. The FDA has issued specific warnings about compounded GLP-1 receptor agonists, including concerns about product quality and patient safety.
Patients should be aware that purchasing tirzepatide from online sources, international pharmacies not licensed in the United States, or suppliers offering "research grade" or powder forms carries significant risks. These products may be counterfeit, contaminated, improperly stored, incorrectly dosed, or contain inactive or harmful ingredients. The FDA actively warns against the use of such products and has issued alerts regarding compounded GLP-1 receptor agonist preparations that may pose health risks.
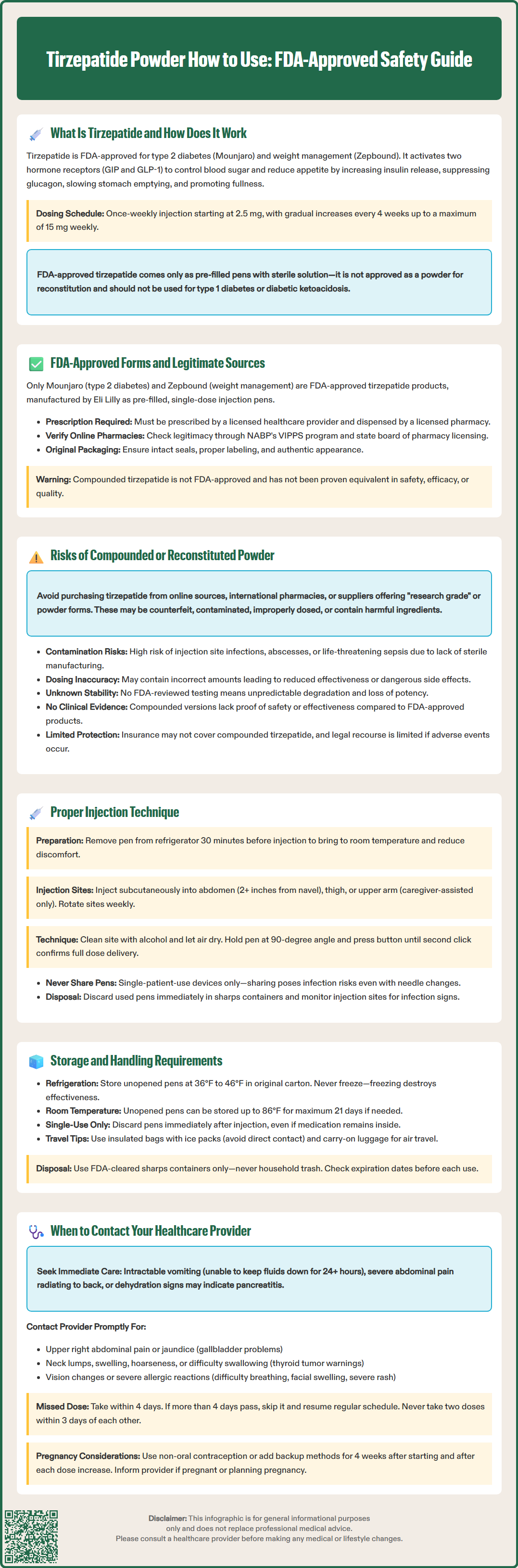
Using compounded or reconstituted tirzepatide powder presents multiple serious health and safety concerns that patients and healthcare providers must understand. Unlike FDA-approved medications that undergo rigorous testing for purity, potency, and sterility, compounded preparations lack this regulatory oversight and quality assurance.
Contamination and Sterility Risks: Reconstituting powder forms of tirzepatide requires sterile technique and appropriate facilities. Improper reconstitution can introduce bacterial contamination, leading to injection site infections, abscesses, or potentially life-threatening systemic infections such as sepsis. Even when performed in compounding pharmacies, the risk of contamination exceeds that of FDA-approved pre-filled pens manufactured under current Good Manufacturing Practice (cGMP) standards.
Dosing Inaccuracy: Compounded tirzepatide may not contain the stated amount of active ingredient, leading to underdosing (reduced efficacy) or overdosing (increased adverse effects). Reconstitution errors by patients or pharmacies can further compound this problem. Tirzepatide has dose-dependent adverse effects; dosing errors can increase the risk of severe gastrointestinal effects, including nausea, vomiting, diarrhea, and potentially dangerous dehydration.
Unknown Ingredients and Stability: Compounded products may contain different inactive ingredients, preservatives, or buffers than FDA-approved formulations. These substitutions can affect drug stability, absorption, and may trigger allergic reactions or other adverse effects. The stability and shelf-life of reconstituted tirzepatide powder have not been established through FDA-reviewed studies, meaning the medication may degrade unpredictably, losing potency or affecting safety.
Lack of Clinical Data: The safety and efficacy data supporting tirzepatide use come exclusively from studies using FDA-approved formulations. There is no clinical evidence demonstrating that compounded versions produce equivalent therapeutic effects or safety profiles. Patients using compounded products are essentially participating in an uncontrolled experiment without informed consent or safety monitoring.
Legal and Insurance Implications: Using non-FDA-approved compounded tirzepatide may void insurance coverage and leave patients without recourse if adverse events occur. If adverse events occur with any medication, including compounded products, they should be reported to the FDA through the MedWatch program and to relevant state pharmacy boards.
For patients using FDA-approved tirzepatide pens (Mounjaro or Zepbound), proper injection technique is essential for medication efficacy, comfort, and safety. Healthcare providers should provide hands-on training before patients self-administer at home.
Preparation Steps: Remove the pen from refrigerated storage 30 minutes before injection to allow it to reach room temperature, which reduces injection discomfort. Wash hands thoroughly with soap and water. Do not attempt to inspect the solution inside the single-dose pen, as these pens are not designed for visual inspection. Gather supplies including the tirzepatide pen, alcohol wipes, and a sharps disposal container.
Injection Site Selection: Tirzepatide should be injected subcutaneously into the abdomen, thigh, or upper arm. The abdomen (except within 2 inches of the navel) is often preferred for self-injection due to ease of access. Upper arm injections should only be administered by a caregiver, not self-administered. Rotate injection sites with each weekly dose to minimize the risk of local reactions and injection site irritation. Do not inject into areas that are tender, bruised, red, hard, or scarred.
Injection Procedure: Clean the selected injection site with an alcohol wipe and allow it to air dry completely. Remove the pen cap. Unlock the pen according to the Instructions for Use. Place the pen firmly against the skin at a 90-degree angle. Press and hold the injection button until you hear the second click or see the indicator showing the dose has been delivered. Remove the pen from the skin after the second click or when the indicator shows the dose is complete. Do not share pens with others, even if the needle has been changed.
Post-Injection Care: A small amount of bleeding or clear fluid at the injection site is normal. Apply gentle pressure with a clean gauze pad if needed, but do not rub the area. Do not apply bandages unless necessary for bleeding control. Monitor the injection site for signs of infection (increasing redness, warmth, swelling, or pus) over the following days. Dispose of the used pen immediately in a sharps container.
Proper storage and handling of tirzepatide are critical to maintain medication stability, potency, and safety. FDA-approved tirzepatide pens have specific storage requirements that must be followed precisely.
Refrigerated Storage: Unopened tirzepatide pens must be stored in a refrigerator at 36°F to 46°F (2°C to 8°C). Store the pens in their original carton to protect them from light. Do not store tirzepatide in the freezer, and do not use pens that have been frozen, even if subsequently thawed. Freezing can damage the protein structure of the medication, rendering it ineffective or potentially harmful. Keep tirzepatide away from the freezer compartment and avoid placing it directly against the back wall of the refrigerator where temperatures may be coldest.
Room Temperature Storage: If needed, an unopened pen may be stored at room temperature (up to 86°F or 30°C) for up to 21 days before use. However, refrigerated storage is preferred when possible. Tirzepatide pens are for single use only and should be discarded immediately after injection, regardless of whether any medication appears to remain in the pen. There is no storage period after first use, as these are single-dose pens.
Handling Precautions: Never leave tirzepatide pens in direct sunlight, in hot vehicles, or near heat sources such as radiators or stoves. Extreme temperatures can degrade the medication. When traveling, transport tirzepatide in an insulated bag with ice packs if refrigeration is unavailable, ensuring the pen does not come into direct contact with ice or ice packs (which could cause freezing). For air travel, carry tirzepatide in carry-on luggage rather than checked baggage, as cargo holds may experience freezing temperatures.
Disposal: Used pens should be disposed of in an FDA-cleared sharps disposal container immediately after use. Never throw pens in household trash or recycling bins. Many communities offer sharps disposal programs or mail-back services. Unused or expired tirzepatide should not be flushed down toilets or poured into drains unless specifically instructed by disposal guidelines. Contact your pharmacy or local waste management authority for proper disposal instructions.
Expiration Dates: Always check the expiration date printed on the pen and carton. Do not use tirzepatide past its expiration date, as potency and safety cannot be guaranteed.
Patients using tirzepatide should maintain regular communication with their healthcare provider and know when to seek medical attention. Certain situations require prompt evaluation to ensure safe and effective treatment.
Severe Gastrointestinal Symptoms: While mild to moderate nausea, vomiting, and diarrhea are common with tirzepatide, especially during dose escalation, severe or persistent symptoms warrant medical attention. Contact your provider if you experience intractable vomiting (unable to keep down fluids for more than 24 hours), severe abdominal pain (particularly if constant or radiating to the back), or signs of dehydration including decreased urination, extreme thirst, dizziness, or confusion. These symptoms may indicate pancreatitis, a serious but rare adverse effect, or severe dehydration requiring intervention.
Gallbladder Problems: Contact your healthcare provider promptly if you experience symptoms of gallbladder disease, including pain in the upper right portion of your abdomen, fever, yellowing of skin or eyes (jaundice), or clay-colored stools. Tirzepatide has been associated with an increased risk of gallbladder-related disorders.
Hypoglycemia Symptoms: Although tirzepatide has a low intrinsic risk of hypoglycemia, patients taking concurrent insulin or sulfonylureas may experience low blood sugar. Seek guidance if you develop symptoms such as shakiness, sweating, rapid heartbeat, confusion, blurred vision, or severe hunger. Your provider may need to adjust doses of other diabetes medications.
Allergic Reactions: Discontinue tirzepatide and seek immediate medical attention if you develop signs of a serious allergic reaction, including difficulty breathing, swelling of the face, lips, tongue, or throat, severe rash, or hives. Injection site reactions (mild redness, itching, or swelling) are usually not serious but should be reported if they worsen or persist.
Thyroid-Related Concerns: Although primarily observed in rodent studies, tirzepatide carries a boxed warning regarding thyroid C-cell tumors. Contact your provider immediately if you develop a lump or swelling in your neck, hoarseness, difficulty swallowing, or shortness of breath. Patients with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 should not use tirzepatide.
Vision Changes: Report any vision changes, particularly if you have pre-existing diabetic retinopathy. Rapid improvement in blood glucose may temporarily worsen retinopathy in some patients.
Kidney Problems: Notify your provider if you experience decreased urination, swelling in legs or feet, or unusual fatigue, as these may indicate kidney function changes, particularly in the setting of dehydration from gastrointestinal adverse effects.
Suicidal Thoughts or Behaviors: For patients using Zepbound for weight management, monitor for new or worsening depression, suicidal thoughts, or unusual changes in mood or behavior. Discontinue Zepbound if you experience suicidal thoughts or behaviors and contact your healthcare provider immediately.
Missed Doses: If you miss a dose, administer it within 4 days after the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. Do not administer two doses within 3 days of each other. If you wish to change the day of weekly administration, you may do so as long as the last dose was administered 3 or more days before.
Pregnancy and Breastfeeding: Inform your provider if you are pregnant, planning to become pregnant, or breastfeeding. Tirzepatide for weight management (Zepbound) is not recommended during pregnancy. Women using oral contraceptives should use a non-oral method or add a backup method for 4 weeks after initiating tirzepatide and for 4 weeks after each dose increase due to potential reduced contraceptive effectiveness.
Adverse Event Reporting: Report any adverse effects to your healthcare provider and to the FDA MedWatch program (www.fda.gov/medwatch or 1-800-FDA-1088).
No, tirzepatide powder is not FDA-approved for patient use. Only pre-filled injection pens (Mounjaro and Zepbound) manufactured by Eli Lilly are approved, as compounded or reconstituted powder forms carry risks of contamination, dosing errors, and unknown ingredient stability.
Inject tirzepatide subcutaneously once weekly into the abdomen, thigh, or upper arm after allowing the pen to reach room temperature. Clean the site with alcohol, place the pen at 90 degrees, press the button until the second click, then dispose immediately in a sharps container.
Store unopened tirzepatide pens in the refrigerator at 36°F to 46°F in the original carton. Never freeze the medication, and if stored at room temperature (up to 86°F), use within 21 days.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.