LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Understanding the difference between semaglutide and GLP-1 is essential for patients and clinicians navigating diabetes and weight management therapies. GLP-1 (glucagon-like peptide-1) is a naturally occurring hormone produced in the intestines that regulates blood sugar and appetite but lasts only 1-2 minutes in the body. Semaglutide is a synthetic medication engineered to mimic GLP-1's effects while remaining active for approximately 7 days, enabling practical once-weekly dosing. This fundamental pharmacokinetic difference transforms a fleeting physiological signal into a powerful therapeutic agent approved by the FDA for type 2 diabetes management and chronic weight management.
Quick Answer: Semaglutide is a synthetic medication engineered to mimic natural GLP-1 hormone but with a 7-day half-life compared to GLP-1's 1-2 minute duration, enabling practical therapeutic use.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone naturally produced by specialized L-cells in the distal small intestine and colon. This endogenous peptide plays a crucial role in glucose homeostasis and metabolic regulation. When food enters the gastrointestinal tract, GLP-1 is released into the bloodstream in response to nutrient intake, particularly carbohydrates and fats.
The physiological actions of native GLP-1 are multifaceted and clinically significant. The hormone stimulates glucose-dependent insulin secretion from pancreatic beta cells, meaning insulin release occurs only when blood glucose levels are elevated. Simultaneously, GLP-1 suppresses glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. Beyond pancreatic effects, GLP-1 slows gastric emptying, which moderates postprandial glucose excursions, and acts on central nervous system receptors to promote satiety and reduce appetite.
Despite these beneficial metabolic effects, native GLP-1 has significant pharmacokinetic limitations that restrict its therapeutic utility. The hormone has an extremely short half-life of approximately 1 to 2 minutes in circulation. This rapid degradation occurs primarily through the enzyme dipeptidyl peptidase-4 (DPP-4) and neutral endopeptidase (NEP 24.11), along with renal clearance. The brief duration of action means that endogenous GLP-1 functions as a meal-related signal rather than providing sustained metabolic control.
Understanding the physiology of natural GLP-1 provides essential context for appreciating why pharmaceutical modifications were necessary to harness its therapeutic potential for chronic disease management, particularly in type 2 diabetes and obesity treatment.
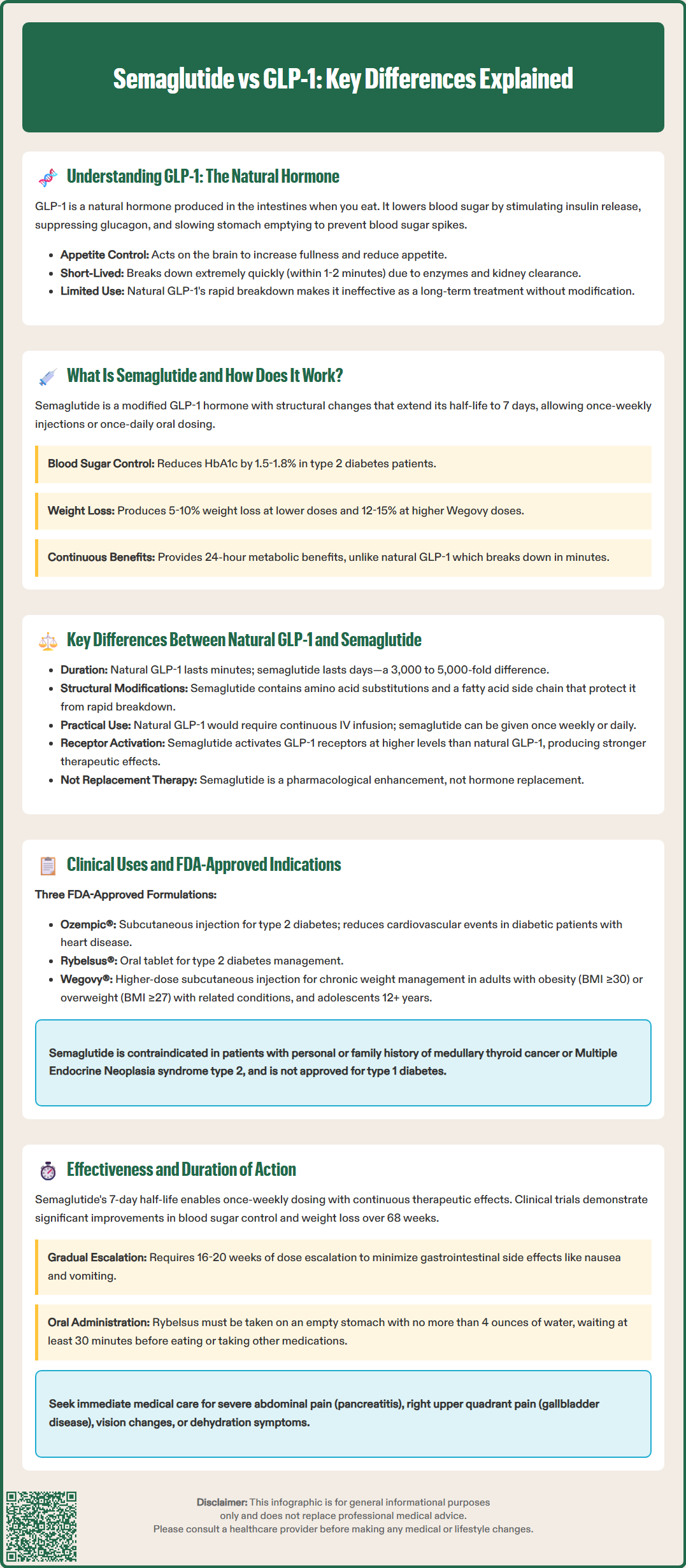
Semaglutide is a synthetic glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed to overcome the pharmacokinetic limitations of native GLP-1. Approved by the FDA for type 2 diabetes management (as Ozempic® and Rybelsus®) and chronic weight management (as Wegovy®), semaglutide represents a significant advancement in GLP-1-based therapeutics. The medication shares approximately 94% structural homology with human GLP-1 but incorporates strategic molecular modifications that dramatically extend its duration of action.
The pharmacological design of semaglutide includes three key structural alterations. First, an amino acid substitution at position 8 (alanine replaced with aminoisobutyric acid) provides resistance to DPP-4 degradation, the primary enzyme responsible for native GLP-1 breakdown. Second, a spacer and C-18 fatty diacid chain are attached to lysine at position 26, enabling reversible binding to albumin in both subcutaneous tissue and circulation. Third, a single amino acid substitution at position 34 (lysine to arginine) prevents unwanted acylation at this position and enhances molecular stability. These modifications result in a half-life of approximately 7 days, enabling once-weekly subcutaneous administration or once-daily oral dosing.
Semaglutide activates the same GLP-1 receptors as the endogenous hormone, producing comparable physiological effects but with sustained duration. The medication enhances glucose-dependent insulin secretion, suppresses inappropriate glucagon release, delays gastric emptying, and reduces appetite through central mechanisms. Clinical studies demonstrate that semaglutide achieves glycemic control with HbA1c reductions of 1.5% to 1.8% at antidiabetic doses (Ozempic 0.5-1.0 mg, Rybelsus 7-14 mg) and produces weight loss ranging from 5-10% in type 2 diabetes and 12-15% with higher doses (Wegovy 2.4 mg) in patients with obesity.
The extended pharmacokinetic profile allows therapeutic GLP-1 receptor activation throughout the day and night, providing continuous metabolic benefits rather than the transient effects of native GLP-1.
The fundamental distinction between natural GLP-1 and semaglutide lies in their pharmacokinetic properties and resultant clinical applicability. Native GLP-1 functions as a rapid-response, meal-related hormone with a half-life measured in minutes, while semaglutide is an engineered therapeutic agent with a half-life measured in days. This approximately 3,000 to 5,000-fold difference in half-life represents the primary pharmacological divergence between the endogenous hormone and its synthetic analog.
Structural and molecular differences include:
Amino acid composition: Semaglutide contains specific substitutions that confer DPP-4 resistance, whereas native GLP-1 is rapidly cleaved by this ubiquitous enzyme
Albumin binding: The fatty acid side chain attached to lysine at position 26 in semaglutide enables prolonged circulation through albumin binding, a property absent in natural GLP-1
Molecular stability: Structural modifications protect semaglutide from enzymatic degradation and renal clearance that rapidly eliminate native GLP-1
From a clinical perspective, natural GLP-1 cannot be administered as a therapeutic agent due to its extremely short half-life, which would require continuous intravenous infusion for sustained effect. While this has been done in research settings, it is not practical for routine clinical therapy. Semaglutide, conversely, provides practical therapeutic administration through once-weekly injection or once-daily oral tablet. The sustained receptor activation achieved by semaglutide produces more pronounced and consistent metabolic effects compared to the pulsatile, meal-related actions of endogenous GLP-1.
Additionally, the magnitude of clinical effects differs substantially. While native GLP-1 contributes to normal glucose regulation, semaglutide produces receptor activation at levels that exceed endogenous postprandial GLP-1 concentrations, generating clinically significant HbA1c reduction and weight loss. The synthetic analog achieves therapeutic concentrations that exceed those of endogenous GLP-1, resulting in more robust appetite suppression and metabolic improvement. These differences underscore that semaglutide is not simply replacement of a deficient hormone but rather pharmacological augmentation of GLP-1 receptor signaling for therapeutic benefit.
Semaglutide has received FDA approval for distinct clinical indications based on formulation and dosing regimen, reflecting its versatility as a GLP-1 receptor agonist. The subcutaneous formulation marketed as Ozempic® is approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Additionally, Ozempic® is indicated to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with type 2 diabetes and established cardiovascular disease. The oral formulation, Rybelsus®, is approved specifically for glycemic management in type 2 diabetes.
Wegovy®, a higher-dose subcutaneous semaglutide formulation, received FDA approval for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity such as hypertension, type 2 diabetes, or dyslipidemia. This indication extends to adolescents aged 12 years and older with BMI ≥95th percentile for age and sex. In 2024, Wegovy® also received FDA approval to reduce the risk of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in adults with established cardiovascular disease and either obesity or overweight.
In contrast, natural GLP-1 has no therapeutic applications as an administered medication due to its pharmacokinetic limitations. The endogenous hormone functions exclusively as a physiological regulator of glucose homeostasis and appetite. While GLP-1 physiology informs the development of therapeutic agents, the native peptide itself cannot be practically administered for disease management.
Current FDA-approved uses for semaglutide include:
Type 2 diabetes management (glycemic control)
Cardiovascular risk reduction in type 2 diabetes with established CVD (Ozempic)
Cardiovascular risk reduction in adults with established CVD and obesity/overweight (Wegovy)
Chronic weight management in obesity or overweight with comorbidities
Clinicians should prescribe semaglutide according to FDA-approved indications and established clinical guidelines, including those from the American Diabetes Association (ADA) and American College of Physicians (ACP). Semaglutide is not indicated for type 1 diabetes. Patient selection should consider contraindications, including personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (boxed warning), and hypersensitivity to semaglutide or any product components.
The effectiveness profiles of natural GLP-1 and semaglutide differ dramatically due to their contrasting pharmacokinetic properties. Native GLP-1 provides transient, meal-related metabolic effects lasting minutes, contributing to normal postprandial glucose regulation but offering no sustained therapeutic impact. Semaglutide, with its 7-day half-life, maintains therapeutic plasma concentrations continuously, producing consistent 24-hour receptor activation and clinically meaningful metabolic improvements.
Clinical trial data demonstrate semaglutide's effectiveness in FDA-approved indications. In the SUSTAIN clinical trial program evaluating subcutaneous semaglutide for type 2 diabetes, once-weekly dosing (0.5 mg or 1.0 mg) reduced HbA1c by 1.5% to 1.8% from baseline, with superior glycemic control compared to specific comparators in head-to-head trials (SUSTAIN-7 vs dulaglutide; SUSTAIN-4 vs insulin glargine). The STEP trial program examining higher-dose semaglutide (2.4 mg weekly) for weight management demonstrated mean weight loss of 12% to 15% over 68 weeks in adults with obesity, significantly exceeding placebo.
The duration of action fundamentally distinguishes these agents' clinical utility. Natural GLP-1's 1- to 2-minute half-life means its effects dissipate rapidly after secretion, providing only momentary metabolic influence. Semaglutide's extended half-life enables:
Sustained glycemic control: Continuous insulin secretion enhancement and glucagon suppression throughout the interdose interval
Persistent appetite suppression: Ongoing central nervous system effects reducing caloric intake
Convenient dosing: Once-weekly administration improving adherence compared to daily or multiple-daily injections
Patients should understand that semaglutide requires several weeks to reach steady-state concentrations after each dose change (approximately 4-5 half-lives), with full clinical effects typically observed after completing the recommended dose titration schedule. For Wegovy, this titration extends over 16-20 weeks. Dose escalation follows a gradual titration schedule to minimize gastrointestinal adverse effects, the most common being nausea, vomiting, and diarrhea.
Clinicians should monitor patients for potential adverse effects including pancreatitis (severe, persistent abdominal pain), gallbladder disease (right upper quadrant pain), diabetic retinopathy complications (especially with rapid glucose improvement), acute kidney injury with dehydration, and hypoglycemia when combined with insulin or sulfonylureas. Patients should be advised to seek prompt medical evaluation for severe or persistent gastrointestinal symptoms, vision changes, unusual neck swelling or hoarseness, or severe constipation with abdominal distention. Rybelsus must be taken on an empty stomach with no more than 4 ounces of water, at least 30 minutes before the first food, beverage, or other oral medications of the day.
No, semaglutide is a synthetic medication that mimics natural GLP-1 but with structural modifications that extend its half-life from 1-2 minutes to approximately 7 days. This allows semaglutide to be administered once weekly for sustained therapeutic effects, whereas natural GLP-1 functions only as a brief meal-related signal.
Natural GLP-1 cannot be used therapeutically because it is rapidly degraded by the enzyme DPP-4 within 1-2 minutes, requiring continuous intravenous infusion for sustained effect. This makes it impractical for routine clinical use, necessitating the development of modified GLP-1 receptor agonists like semaglutide.
Semaglutide is FDA-approved for type 2 diabetes management (Ozempic, Rybelsus), cardiovascular risk reduction in patients with type 2 diabetes and established cardiovascular disease (Ozempic), chronic weight management in obesity or overweight with comorbidities (Wegovy), and cardiovascular risk reduction in adults with established cardiovascular disease and obesity or overweight (Wegovy).
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.