LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

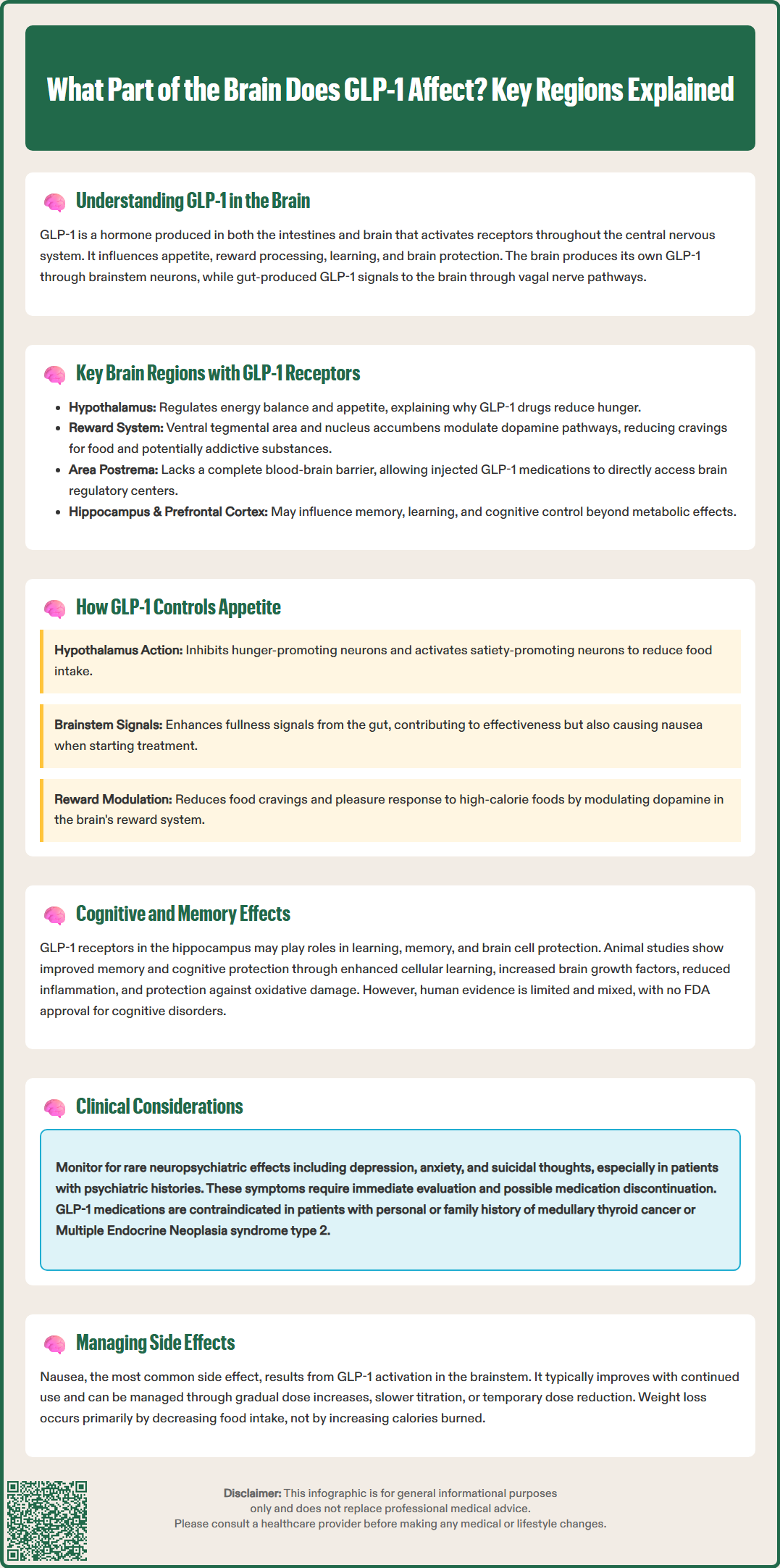
GLP-1 receptor agonists like semaglutide (Ozempic, Wegovy) and liraglutide (Victoza, Saxenda) work throughout multiple brain regions to reduce appetite and support weight loss. While GLP-1 is primarily known for managing blood sugar in type 2 diabetes, its effects on the central nervous system are equally important. GLP-1 receptors are distributed across the hypothalamus, brainstem, reward centers, and memory regions, influencing hunger signals, food preferences, and potentially cognitive function. Understanding which brain areas GLP-1 affects helps explain both the therapeutic benefits and common side effects of these FDA-approved medications.
Quick Answer: GLP-1 affects multiple brain regions including the hypothalamus, brainstem, mesolimbic reward system, hippocampus, and cortical areas involved in appetite regulation, reward processing, and cognitive function.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone primarily produced by intestinal L-cells in response to nutrient intake. While traditionally recognized for its role in glucose homeostasis and insulin secretion, GLP-1 exerts significant effects on the central nervous system through activation of GLP-1 receptors (GLP-1R) distributed throughout various brain regions.
GLP-1 crosses the blood-brain barrier in limited amounts, but the brain also produces its own GLP-1 through neurons in the nucleus tractus solitarius (NTS) of the brainstem. These central GLP-1-producing neurons project widely throughout the brain, creating an extensive network that influences multiple physiological processes including appetite regulation, reward processing, learning, and neuroprotection. Gut-brain signaling via vagal pathways provides another important route for GLP-1's central nervous system effects.
The therapeutic relevance of brain GLP-1 activity has expanded considerably with the widespread use of GLP-1 receptor agonists such as semaglutide (Ozempic, Wegovy) and liraglutide (Victoza, Saxenda). These medications, originally developed for type 2 diabetes management, produce substantial weight loss partly through central nervous system mechanisms, though most glucose-lowering effects occur through peripheral actions.
Current evidence suggests GLP-1 receptor activation in the brain contributes to reduced food intake, altered food preferences, improved glycemic control, and potentially neuroprotective effects. The FDA-approved labels for GLP-1 receptor agonists acknowledge effects on satiety but emphasize their primary metabolic actions. Ongoing research investigates whether GLP-1-based therapies may offer benefits for neurodegenerative conditions, though such applications remain investigational and are not FDA-approved.
GLP-1 receptors demonstrate widespread but selective distribution throughout the central nervous system. Based primarily on preclinical studies with limited human confirmation, GLP-1 receptors appear enriched in regions involved in autonomic control, feeding behavior, reward processing, and cognitive function. Understanding this anatomical distribution provides insight into the diverse effects of GLP-1 receptor agonists.
Key brain regions with GLP-1 receptor expression include:
Hypothalamus: Multiple hypothalamic nuclei express GLP-1 receptors, particularly the arcuate nucleus (ARC), paraventricular nucleus (PVN), and ventromedial hypothalamus (VMH). These areas integrate metabolic signals and regulate energy balance, making them critical for GLP-1's effects on appetite and glucose homeostasis.
Brainstem: The area postrema, nucleus tractus solitarius, and dorsal motor nucleus of the vagus contain both GLP-1-producing neurons and GLP-1 receptors. This region processes visceral sensory information and coordinates autonomic responses to feeding.
Mesolimbic reward system: The ventral tegmental area (VTA) and nucleus accumbens express GLP-1 receptors, positioning GLP-1 to modulate dopaminergic reward pathways that influence food reward and potentially addictive behaviors.
Hippocampus: GLP-1 receptor expression occurs in hippocampal regions involved in learning and memory formation, suggesting roles beyond metabolic regulation.
Cortical areas: The prefrontal cortex and insular cortex show GLP-1 receptor presence, potentially contributing to cognitive control of eating and interoceptive awareness.
The circumventricular organs, including the area postrema, lack a complete blood-brain barrier, allowing circulating GLP-1 and GLP-1 receptor agonists more direct access to these critical regulatory centers. This anatomical feature partially explains how peripherally administered GLP-1 medications exert central effects despite limited overall brain penetration.
The appetite-suppressing effects of GLP-1 receptor agonists represent one of their most clinically significant brain actions. These effects occur through coordinated activity across multiple interconnected brain regions that regulate hunger, satiety, and food reward.
In the hypothalamus, GLP-1 receptor activation influences key neuronal populations that control energy balance. The arcuate nucleus contains two opposing neuronal types: appetite-stimulating neurons (expressing neuropeptide Y and agouti-related peptide) and appetite-suppressing neurons (expressing pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript). GLP-1 receptor activation inhibits hunger-promoting neurons while stimulating satiety-promoting neurons, creating a coordinated anorexigenic effect. The paraventricular nucleus integrates these signals and coordinates downstream responses including reduced food intake.
The brainstem provides another critical site for GLP-1's appetite effects. The area postrema and nucleus tractus solitarius receive vagal afferent signals from the gastrointestinal tract about nutrient status and gastric distension. GLP-1 receptor activation in these regions enhances satiety signaling and may contribute to the nausea that some patients experience with GLP-1 medications, particularly during dose escalation. This adverse effect typically diminishes with continued treatment but represents a common reason for medication discontinuation.
The mesolimbic reward system represents a third mechanism through which GLP-1 affects eating behavior. Based primarily on animal studies with supporting human imaging data, GLP-1 receptor activation in the ventral tegmental area and nucleus accumbens appears to modulate dopamine signaling in response to palatable foods, potentially decreasing the rewarding properties of high-calorie foods. Patients often report reduced food cravings and decreased interest in previously preferred foods, which may reflect altered reward processing.
Clinically, these combined mechanisms produce dose-dependent reductions in appetite and food intake. According to FDA prescribing information, weight loss with GLP-1 receptor agonists occurs primarily through reduced energy intake rather than increased energy expenditure.
Beyond metabolic regulation, GLP-1 receptors in cognitive brain regions suggest potential roles in learning, memory, and neuroprotection. The hippocampus, a structure critical for memory formation and spatial learning, expresses GLP-1 receptor levels, and preclinical studies demonstrate that GLP-1 receptor activation enhances synaptic plasticity and neurogenesis in this region.
Animal research indicates that GLP-1 receptor agonists improve performance on memory tasks and protect against experimentally induced cognitive deficits. Proposed mechanisms include enhanced long-term potentiation (a cellular correlate of learning), increased brain-derived neurotrophic factor expression, reduced neuroinflammation, and protection against oxidative stress. These findings have generated interest in whether GLP-1-based therapies might benefit neurodegenerative conditions such as Alzheimer's disease.
Human evidence remains more limited and mixed. Some observational studies of patients with type 2 diabetes suggest that GLP-1 receptor agonist use associates with reduced dementia risk compared with other glucose-lowering medications, but these findings require cautious interpretation due to potential confounding factors. Small clinical trials have explored GLP-1 receptor agonists in Parkinson's disease and Alzheimer's disease with modest signals of potential benefit, but definitive evidence is lacking.
Currently, there is no FDA-approved indication for GLP-1 receptor agonists in cognitive disorders or neuroprotection. The American Diabetes Association guidelines do not recommend selecting GLP-1 medications specifically for cognitive benefits. Clinicians should counsel patients that while preclinical data appear promising, cognitive enhancement remains an investigational application requiring further study.
Patients occasionally report subjective cognitive changes (both improvements and impairments) when starting GLP-1 medications, though systematic data on such effects are limited. Any concerning cognitive symptoms warrant clinical evaluation to exclude other causes, and the risk-benefit balance of continuing GLP-1 therapy should be individually assessed.
Understanding GLP-1's brain effects has important implications for clinical practice, informing both therapeutic applications and adverse effect management. The central nervous system actions of GLP-1 receptor agonists contribute substantially to their clinical benefits but also account for common tolerability issues.
Therapeutic considerations include recognizing that weight loss with GLP-1 medications results from both peripheral metabolic effects and central appetite suppression. Patients should receive counseling that reduced appetite represents an expected pharmacological effect rather than a concerning symptom. The dose-dependent nature of appetite suppression supports the gradual dose escalation protocols recommended in FDA labeling, which help minimize gastrointestinal adverse effects including nausea and vomiting.
The potential for altered food reward processing may benefit patients with binge eating patterns or food addiction-like behaviors, though GLP-1 medications lack specific FDA approval for eating disorders. Some patients report decreased alcohol consumption while taking GLP-1 receptor agonists, possibly reflecting effects on reward pathways, but this remains an area of ongoing investigation without established clinical recommendations.
Adverse effect management requires awareness of centrally mediated symptoms. Nausea, the most common adverse effect, likely reflects GLP-1 receptor activation in brainstem areas controlling emesis. Management strategies include slower dose titration and temporary dose reduction if needed. For oral semaglutide (Rybelsus), patients must take it upon waking with no more than 4 ounces of water, at least 30 minutes before any food, beverage, or other oral medications, as taking it with food significantly reduces absorption.
Rare neuropsychiatric effects including mood changes, suicidal ideation, and anxiety have been reported with GLP-1 medications. The FDA prescribing information for weight management formulations (Wegovy, Saxenda) includes warnings about monitoring for suicidal thoughts or behaviors. Prescribers should monitor mental health, particularly in patients with psychiatric histories. Any emergence of depression or suicidal thoughts requires prompt psychiatric evaluation and consideration of medication discontinuation.
Patient safety guidance should include:
Education about expected appetite changes and strategies for maintaining adequate nutrition
Monitoring for excessive weight loss or signs of malnutrition
Assessment of mental health at follow-up visits
Clear instructions to report severe or persistent nausea, vomiting, or neuropsychiatric symptoms
Awareness that cognitive effects remain investigational and should not influence prescribing decisions
Reporting severe persistent abdominal pain (pancreatitis), right upper quadrant pain/jaundice (gallbladder disease), severe abdominal distension with inability to pass gas or stool (possible ileus), persistent vomiting or signs of dehydration, and new/worsening vision changes (retinopathy)
Understanding increased hypoglycemia risk when combined with insulin or sulfonylureas
GLP-1 receptor agonists are contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2, and should be used with caution in patients with severe gastrointestinal disease, including gastroparesis.
Clinicians should maintain realistic expectations about GLP-1 brain effects, emphasizing established benefits for glycemic control and weight management while acknowledging that applications for cognitive enhancement or neuroprotection remain unproven. Current prescribing should align with FDA-approved indications and evidence-based guidelines from organizations such as the American Diabetes Association and American College of Physicians.
The hypothalamus, particularly the arcuate nucleus and paraventricular nucleus, plays the most critical role in GLP-1's appetite suppression by inhibiting hunger-promoting neurons and activating satiety pathways. The brainstem area postrema and nucleus tractus solitarius also contribute significantly by processing gut signals about fullness.
Yes, GLP-1 receptors in the ventral tegmental area and nucleus accumbens modulate dopamine signaling in the mesolimbic reward system. This may reduce the rewarding properties of high-calorie foods and decrease food cravings, which patients often report when taking GLP-1 medications.
While GLP-1 receptors are present in the hippocampus and preclinical studies suggest potential cognitive benefits, there is currently no FDA-approved indication for GLP-1 medications in cognitive disorders or neuroprotection. Any cognitive benefits remain investigational and require further clinical study.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.