LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Balanced hormone health tirzepatide represents a dual-action medication that influences multiple metabolic pathways through its effects on incretin hormones. FDA-approved as Mounjaro for type 2 diabetes and Zepbound for chronic weight management, tirzepatide targets both GIP and GLP-1 receptors to improve glucose regulation, reduce appetite, and promote weight loss. While the medication affects various hormonal systems through its metabolic actions, it is specifically indicated for diabetes management and weight reduction in appropriate patients. Understanding how tirzepatide works and its broader metabolic effects helps clinicians and patients make informed treatment decisions based on established clinical evidence.
Quick Answer: Tirzepatide is a dual GIP/GLP-1 receptor agonist that improves metabolic balance by enhancing glucose-dependent insulin secretion, reducing appetite, and promoting weight loss in patients with type 2 diabetes or obesity.
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (under the brand name Mounjaro) and chronic weight management (as Zepbound). This medication targets two key incretin hormone pathways simultaneously.
The drug works by mimicking naturally occurring incretin hormones that regulate glucose metabolism and appetite. When administered subcutaneously once weekly, tirzepatide binds to both GIP and GLP-1 receptors on pancreatic beta cells, enhancing glucose-dependent insulin secretion. This means insulin release occurs primarily when blood glucose levels are elevated, reducing the risk of hypoglycemia when used as monotherapy (though this risk increases when combined with insulin or sulfonylureas). Simultaneously, tirzepatide suppresses inappropriate glucagon secretion from pancreatic alpha cells, further improving glycemic control.
Beyond pancreatic effects, tirzepatide acts on receptors in the central nervous system to reduce appetite and food intake, contributing to the weight loss observed in clinical trials. The medication also slows gastric emptying, which helps moderate postprandial glucose excursions and promotes satiety. These combined mechanisms create a comprehensive metabolic effect that extends beyond glucose lowering.
Tirzepatide is available in escalating doses (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg) to allow gradual titration, minimizing gastrointestinal side effects while optimizing therapeutic response. The pharmacokinetic profile supports once-weekly dosing, with a half-life of approximately five days. According to the FDA prescribing information, no dose adjustment is required for patients with renal or hepatic impairment.
Tirzepatide exerts broad effects on the endocrine system beyond its primary incretin actions, influencing multiple hormonal pathways involved in metabolic homeostasis. The medication's effects on glucose metabolism represent a cornerstone of its benefits. By enhancing insulin secretion in a glucose-dependent manner and through weight loss-mediated improvements in insulin resistance, tirzepatide helps restore more physiologic glucose regulation in patients with type 2 diabetes.
Weight loss associated with tirzepatide treatment triggers secondary hormonal changes that contribute to improved metabolic balance. Adipose tissue reduction typically leads to changes in circulating adipokines, with decreases in leptin and increases in adiponectin levels. These shifts in adipokine profiles are associated with improvements in metabolic function. Additionally, significant weight loss can influence metabolic parameters in individuals with obesity-related conditions.
The medication's effects on appetite regulation involve complex neuroendocrine pathways. GLP-1 receptor activation in hypothalamic regions modulates appetite-regulating hormones and neural circuits, reducing hunger signals and increasing satiety. While tirzepatide does not directly target thyroid function, substantial weight loss may affect thyroid hormone requirements in patients on thyroid replacement therapy, as is generally observed with significant weight changes.
Patients with polycystic ovary syndrome (PCOS) may experience indirect benefits from tirzepatide through weight reduction and subsequent improvements in insulin resistance, though this is not an FDA-approved indication and specific studies in this population remain limited. Clinicians should recognize that while tirzepatide influences metabolic parameters, it is not a hormone replacement therapy and should not be used for general "hormone balancing" outside its approved indications for type 2 diabetes and weight management.
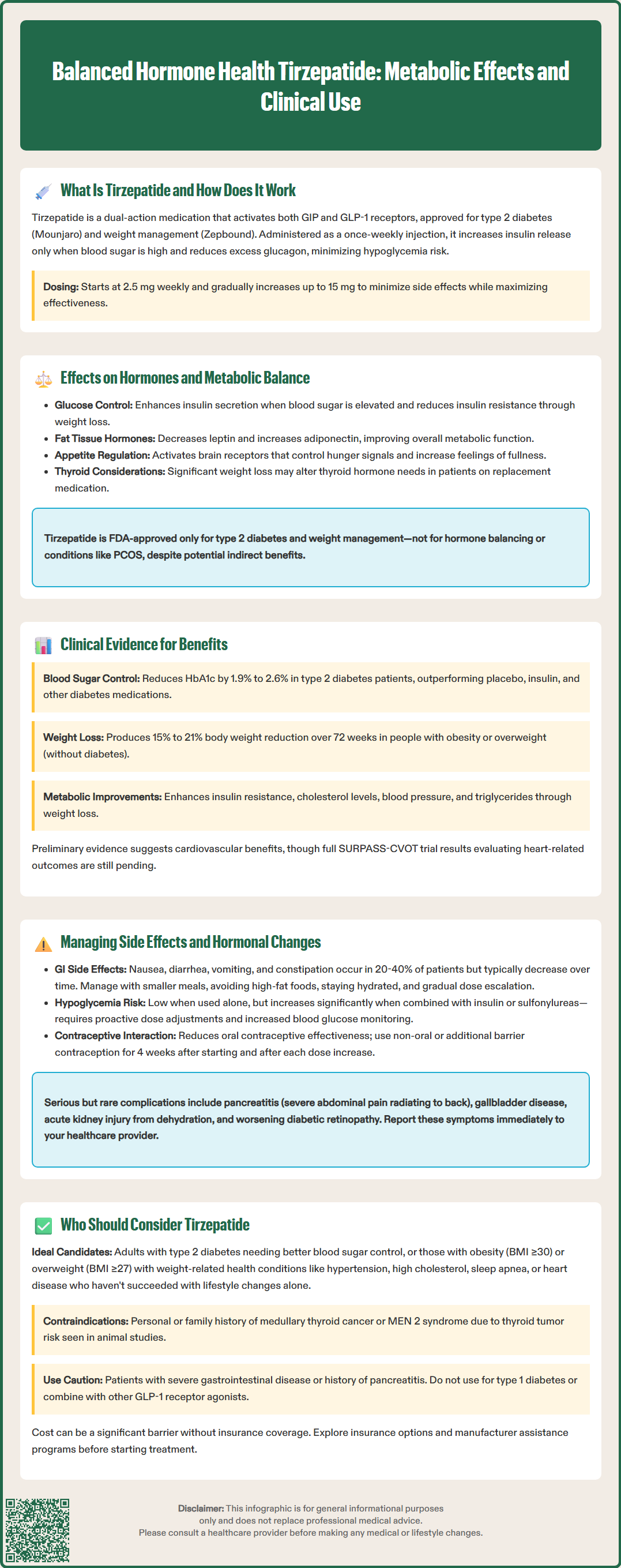
The SURPASS clinical trial program provides robust evidence for tirzepatide's metabolic effects in patients with type 2 diabetes. Across multiple trials, tirzepatide demonstrated superior glycemic control compared to placebo, insulin, and other GLP-1 receptor agonists, with HbA1c reductions ranging from 1.9% to 2.6% depending on dose. These improvements reflect enhanced insulin secretion and glucose regulation, fundamental adaptations in diabetes management.
Weight loss outcomes from the SURMOUNT trials further illustrate tirzepatide's metabolic impact. In the SURMOUNT-1 trial involving adults with obesity or overweight without diabetes, participants achieved mean weight reductions of approximately 15% to 21% over 72 weeks, depending on dose. This substantial weight loss is associated with favorable changes in metabolic parameters, including improvements in markers of insulin resistance (HOMA-IR), and beneficial shifts in lipid profiles.
Cardiovascular outcome data are still emerging. The SURPASS-CVOT trial is ongoing to evaluate cardiovascular outcomes, but preliminary evidence indicates improvements in cardiovascular risk factors including blood pressure and triglycerides. Secondary outcomes in clinical trials have also shown reductions in markers of hepatic steatosis. These changes reflect improved metabolic parameters associated with weight loss and improved glycemic control.
Subgroup analyses have examined tirzepatide's effects across different patient populations, including those with varying degrees of insulin resistance and obesity. Consistent benefits across subgroups support the medication's broad metabolic effects. However, it is important to note that there is no FDA indication for tirzepatide specifically for "hormone balancing" outside its approved uses for type 2 diabetes and weight management. Clinicians should base treatment decisions on established indications and individual patient characteristics rather than unproven hormonal claims.
Gastrointestinal side effects represent the most common adverse events with tirzepatide, occurring in 20% to 40% of patients depending on dose. Nausea, diarrhea, vomiting, constipation, and abdominal discomfort typically emerge during dose escalation and often diminish with continued treatment. These effects result from the medication's action on GLP-1 receptors in the gastrointestinal tract, slowing gastric emptying and affecting gut motility. Gradual dose titration, starting at 2.5 mg weekly and increasing every four weeks as tolerated, helps minimize these symptoms.
Patients should receive counseling on practical strategies to manage gastrointestinal effects: eating smaller, more frequent meals; avoiding high-fat and heavily processed foods; staying well-hydrated; and not lying down immediately after eating. If symptoms persist or worsen, clinicians may consider temporary dose reduction or extended time at a lower dose before further escalation. Severe, persistent vomiting warrants medical evaluation to assess for dehydration and electrolyte disturbances.
Hypoglycemia risk remains low with tirzepatide monotherapy due to its glucose-dependent mechanism, but increases when combined with insulin or sulfonylureas. Patients on these combinations require proactive clinician-directed dose adjustments of concomitant medications, particularly insulin, to prevent hypoglycemic episodes. Blood glucose monitoring should be intensified during treatment initiation and dose changes.
Important safety considerations include: hypersensitivity reactions (discontinue if suspected); thyroid C-cell tumors (boxed warning based on rodent studies); acute pancreatitis (severe abdominal pain radiating to back); gallbladder disease (right upper quadrant pain); acute kidney injury (typically secondary to dehydration); and diabetic retinopathy complications in patients with pre-existing retinopathy. Patients should promptly report severe abdominal pain, visual changes, or signs of dehydration.
Tirzepatide reduces the exposure of oral contraceptives; patients should use non-oral or additional barrier contraception for 4 weeks after initiation and each dose escalation. Women planning pregnancy should discuss discontinuation timing with their healthcare provider. Weight loss may increase fertility in some individuals with obesity-related anovulation. Regular monitoring of renal function is recommended, particularly in patients with pre-existing kidney disease or those experiencing significant gastrointestinal symptoms.
Tirzepatide is FDA-approved for two specific indications: as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes, and for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. Ideal candidates include patients with type 2 diabetes who have not achieved adequate glycemic control with metformin or other first-line agents, particularly those who would benefit from concurrent weight loss.
Patients with obesity or overweight and metabolic complications—such as hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease—represent appropriate candidates for tirzepatide's weight management indication. The medication offers particular value for individuals who have struggled with lifestyle modifications alone or who have not achieved sufficient weight loss with other pharmacologic interventions. The American Diabetes Association (ADA) Standards of Care support considering GLP-1 receptor agonists, including dual agonists like tirzepatide, early in the treatment algorithm for type 2 diabetes, especially in patients with established cardiovascular disease, chronic kidney disease, or those requiring significant weight reduction.
Contraindications include personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), as tirzepatide carries a boxed warning regarding thyroid C-cell tumors observed in rodent studies. Patients with a history of severe gastrointestinal disease, including gastroparesis, may not tolerate the medication well. Caution is warranted in patients with pancreatitis history, though prior pancreatitis is not an absolute contraindication if the underlying cause has been addressed. No dose adjustment is required for patients with renal or hepatic impairment.
Cost and insurance coverage represent practical considerations, as tirzepatide is expensive without insurance support. Patients should discuss coverage options and potential assistance programs with their healthcare team. Shared decision-making should incorporate discussion of treatment goals, potential benefits and risks, lifestyle modification commitment, and long-term treatment plans. Tirzepatide is not appropriate for type 1 diabetes, and it should not be used with other GLP-1 receptor agonists. Referral pathways may include endocrinology, diabetes self-management education and support (DSMES), registered dietitians, and bariatric medicine specialists as appropriate for complex cases or when treatment goals are not met with standard approaches.
Tirzepatide is not a hormone replacement therapy but influences metabolic hormones indirectly through its dual GIP/GLP-1 receptor actions, improving insulin secretion, reducing glucagon, and affecting appetite-regulating pathways. It is FDA-approved specifically for type 2 diabetes and weight management, not for general hormone balancing.
Glycemic improvements typically begin within weeks of starting tirzepatide, while significant weight loss and broader metabolic changes develop over several months with gradual dose escalation. Clinical trials demonstrated maximal effects at 72 weeks with doses titrated every four weeks as tolerated.
While tirzepatide may indirectly benefit PCOS through weight reduction and improved insulin resistance, this is not an FDA-approved indication and specific studies in PCOS populations remain limited. Treatment decisions should be based on established indications for type 2 diabetes and weight management rather than unproven hormonal claims.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.