LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible
Do weight loss injections lower cholesterol? Yes, GLP-1 receptor agonists like semaglutide (Wegovy) and tirzepatide (Zepbound) reduce cholesterol levels alongside promoting weight loss. Clinical trials demonstrate these medications decrease triglycerides by 15-25%, lower LDL cholesterol by 3-7%, and modestly increase HDL cholesterol. These lipid improvements occur through both weight reduction and direct metabolic effects on lipoprotein metabolism. While not replacements for statin therapy, weight loss injections offer complementary cardiovascular benefits for patients with obesity and dyslipidemia when combined with lifestyle modification and standard medical care.
Quick Answer: Weight loss injections, specifically GLP-1 receptor agonists, do lower cholesterol by reducing triglycerides 15-25%, decreasing LDL cholesterol 3-7%, and modestly increasing HDL cholesterol.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Weight loss injections, primarily glucagon-like peptide-1 (GLP-1) receptor agonists such as semaglutide (Wegovy) and tirzepatide (Zepbound), work through multiple physiological mechanisms to promote weight reduction. These medications mimic naturally occurring incretin hormones that regulate glucose metabolism and appetite control.
The primary mechanism involves binding to GLP-1 receptors in the pancreas, brain, and gastrointestinal tract. In the pancreas, these agents enhance glucose-dependent insulin secretion while suppressing inappropriate glucagon release, improving glycemic control with a low risk of hypoglycemia when used alone (though this risk increases when combined with insulin or sulfonylureas). Centrally, GLP-1 receptor agonists act on hypothalamic appetite centers to increase satiety and reduce food intake. They also slow gastric emptying, prolonging the sensation of fullness after meals.
Tirzepatide represents a dual agonist, targeting both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, which may explain its enhanced efficacy in clinical trials. The combined effect produces substantial weight loss—typically about 15% of body weight over 68 weeks with semaglutide 2.4 mg (Wegovy), and up to 22% with tirzepatide 15 mg (Zepbound).
These medications are administered via subcutaneous injection, usually weekly, and require dose titration to minimize gastrointestinal adverse effects. Common side effects include nausea, vomiting, diarrhea, and constipation, which generally diminish over time. The FDA has approved Wegovy (semaglutide 2.4 mg) and Zepbound (tirzepatide) for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. Important contraindications include personal or family history of medullary thyroid carcinoma, multiple endocrine neoplasia syndrome type 2, and pregnancy. Caution is advised in patients with severe gastrointestinal disease, including gastroparesis.
The relationship between body weight and lipid metabolism is well-established in cardiovascular medicine. Excess adiposity, particularly visceral fat, contributes to dyslipidemia through multiple pathways involving insulin resistance, increased hepatic lipogenesis, and altered lipoprotein metabolism.
Obesity typically produces an atherogenic lipid profile characterized by elevated triglycerides, reduced high-density lipoprotein cholesterol (HDL-C), and increased small, dense low-density lipoprotein (LDL) particles. Visceral adipose tissue releases free fatty acids directly into the portal circulation, increasing hepatic very-low-density lipoprotein (VLDL) production. This metabolic cascade elevates triglyceride levels and promotes the formation of small, dense LDL particles—the most atherogenic LDL subtype.
Clinical studies consistently demonstrate that intentional weight loss improves lipid profiles. Research suggests that each 10-kilogram reduction in body weight typically decreases total cholesterol by approximately 5-8 mg/dL and LDL cholesterol by 5-7 mg/dL. Triglycerides show even greater improvement, with reductions of 15-20 mg/dL per 10-kilogram weight loss. HDL cholesterol may initially decrease during active weight loss but generally increases with weight maintenance.
The magnitude of lipid improvement correlates with the degree of weight reduction and varies by weight loss method. While lifestyle modification, pharmacotherapy, and bariatric surgery all improve lipid profiles, the mechanisms and magnitude may differ based on the intervention and individual metabolic factors. Beyond simple weight reduction, decreased visceral adiposity specifically improves insulin sensitivity, reduces systemic inflammation, and normalizes adipokine secretion, all contributing to favorable lipid changes. Current ACC/AHA guidelines also emphasize non-HDL cholesterol and apolipoprotein B as important targets for cardiovascular risk reduction. These mechanisms explain why even modest weight loss (5-10% of initial body weight) produces clinically meaningful cardiovascular risk reduction.
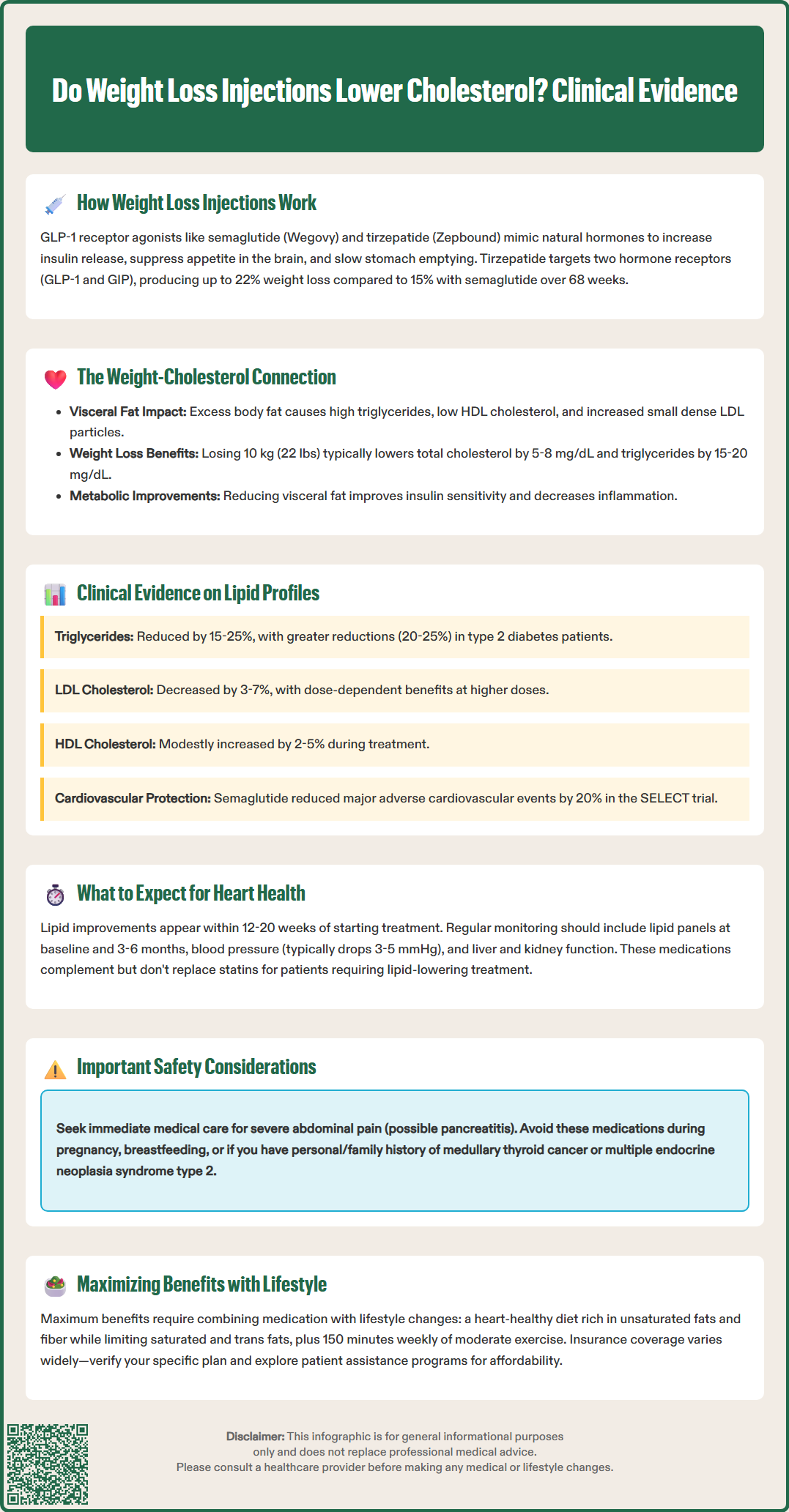
Robust clinical trial data demonstrate that GLP-1 receptor agonists produce favorable effects on lipid profiles that may include mechanisms beyond those attributable to weight loss alone. The STEP (Semaglutide Treatment Effect in People with obesity) trials provide comprehensive evidence for semaglutide's lipid effects, while the SURMOUNT trials establish similar benefits for tirzepatide.
In the STEP 1 trial, participants receiving semaglutide 2.4 mg weekly experienced significant reductions in triglycerides (mean decrease of 15-20%) and total cholesterol compared with placebo. LDL cholesterol decreased by approximately 5-7 mg/dL, while HDL cholesterol showed modest increases. The STEP 2 trial, conducted in patients with type 2 diabetes, demonstrated even greater triglyceride reductions (averaging 20-25%), likely reflecting the baseline metabolic dysfunction in this population.
Tirzepatide trials show comparable or superior lipid benefits. The SURMOUNT-1 study reported dose-dependent triglyceride reductions of 15-25% with the highest doses (10-15 mg weekly), alongside LDL cholesterol decreases of 6-10%. Notably, these improvements occurred early in treatment and persisted throughout the study duration.
Key lipid changes with GLP-1 receptor agonists include:
Triglycerides: 15-25% reduction
Total cholesterol: 3-8% reduction
LDL cholesterol: 3-7% reduction
HDL cholesterol: 2-5% increase
Non-HDL cholesterol: 5-10% reduction
The SELECT cardiovascular outcomes trial demonstrated that semaglutide 2.4 mg reduced major adverse cardiovascular events by 20% in patients with established cardiovascular disease who were receiving standard background care. This suggests that GLP-1 receptor agonists may confer cardiovascular protection through multiple mechanisms, potentially including anti-inflammatory effects, blood pressure reduction, and vascular benefits, in addition to lipid improvements. Importantly, these medications do not replace statin therapy in patients requiring lipid-lowering treatment but may provide complementary benefits in comprehensive cardiovascular risk management.
Patients considering weight loss injections for cardiovascular risk reduction should understand both the realistic benefits and the comprehensive approach required for optimal outcomes. These medications represent one component of cardiovascular risk management, not a standalone solution.
Lipid improvements typically become apparent within 12-20 weeks of initiating therapy, paralleling weight loss trajectory. Patients should expect gradual changes rather than dramatic immediate effects. Healthcare providers typically monitor lipid panels at baseline and after 3-6 months to assess response, with subsequent monitoring individualized based on clinical context. The American College of Cardiology recommends continuing baseline statin therapy in patients with established indications, as GLP-1 receptor agonists complement but do not replace proven lipid-lowering medications.
Patient monitoring should include:
Baseline lipid panel, hemoglobin A1c, and liver function tests
Blood pressure monitoring (GLP-1 agents typically reduce systolic BP by 3-5 mmHg)
Assessment for gastrointestinal adverse effects during dose titration
Evaluation for contraindications (personal or family history of medullary thyroid carcinoma, multiple endocrine neoplasia syndrome type 2)
Monitoring for gallbladder disease (increased risk with rapid weight loss)
In patients with diabetes and retinopathy, monitoring for worsening eye symptoms, particularly with rapid glycemic improvement
Renal function assessment, especially if severe gastrointestinal symptoms cause dehydration
Patients should seek immediate medical attention for severe abdominal pain, which may indicate pancreatitis—a rare but serious adverse effect. Persistent nausea, vomiting, or diarrhea warrant dose adjustment or temporary discontinuation. These medications should be avoided during pregnancy and breastfeeding.
Insurance coverage varies significantly. As of 2024, Medicare Part D may cover Wegovy for cardiovascular risk reduction in eligible beneficiaries with established cardiovascular disease, though coverage varies by plan. Commercial insurance and Medicaid coverage also varies widely. Patients should verify coverage and explore patient assistance programs. Treatment duration remains individualized; current evidence supports long-term use, as weight regain and metabolic deterioration typically occur upon discontinuation. Shared decision-making between patients and clinicians should weigh cardiovascular benefits against cost, adverse effects, and individual treatment goals.
Lipid improvements typically become apparent within 12-20 weeks of initiating GLP-1 receptor agonist therapy, paralleling the weight loss trajectory. Healthcare providers usually monitor lipid panels at baseline and after 3-6 months to assess treatment response.
No, weight loss injections do not replace statin therapy in patients with established indications for lipid-lowering treatment. The American College of Cardiology recommends continuing baseline statin therapy, as GLP-1 receptor agonists provide complementary cardiovascular benefits rather than serving as statin substitutes.
Triglycerides show the greatest improvement with GLP-1 receptor agonists, decreasing by 15-25%. LDL cholesterol decreases by 3-7%, total cholesterol reduces by 3-8%, and HDL cholesterol modestly increases by 2-5%, with additional reductions in non-HDL cholesterol of 5-10%.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.