LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

How do weight loss injections help you lose weight? These prescription medications work by mimicking natural hormones that regulate appetite and digestion. Administered as weekly or daily subcutaneous injections, they belong primarily to the GLP-1 receptor agonist class. By targeting brain appetite centers, slowing stomach emptying, and enhancing satiety signals, these medications help reduce caloric intake naturally. FDA-approved options like semaglutide, tirzepatide, and liraglutide are prescribed alongside lifestyle modifications for adults and adolescents with obesity or overweight conditions with comorbidities. Understanding their mechanisms, expected outcomes, and safety profiles helps patients and clinicians make informed treatment decisions.
Quick Answer: Weight loss injections work by activating GLP-1 receptors that reduce appetite, increase fullness, and slow gastric emptying, leading to decreased caloric intake and sustained weight reduction.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Weight loss injections represent a class of prescription medications administered subcutaneously (under the skin) to support significant and sustained weight reduction in eligible individuals with obesity or overweight conditions. These medications belong primarily to a drug class called glucagon-like peptide-1 (GLP-1) receptor agonists, which were originally developed to manage type 2 diabetes but have demonstrated remarkable efficacy in promoting weight loss.
These injections work by mimicking naturally occurring hormones in the body that regulate appetite, food intake, and blood sugar levels. When administered, they interact with specific receptors in the brain, gastrointestinal tract, and pancreas to create a coordinated physiological response that reduces hunger, increases feelings of fullness, and slows gastric emptying. This multi-targeted approach addresses several mechanisms that contribute to weight gain and difficulty losing weight.
Most weight loss injections are self-administered weekly (such as semaglutide and tirzepatide) or daily (such as liraglutide) using pre-filled pens similar to insulin delivery devices. The medications are prescribed as part of a comprehensive weight management program that includes dietary modifications, increased physical activity, and behavioral counseling. They are not intended as standalone treatments or quick fixes, but rather as pharmacological tools to support lifestyle changes in individuals who have struggled to achieve meaningful weight loss through diet and exercise alone.
The FDA has approved several weight loss injections after rigorous clinical trials demonstrated both safety and efficacy. These medications require a prescription and ongoing medical supervision to monitor response, manage side effects, and adjust treatment as needed. Importantly, patients should only use FDA-approved products prescribed by their healthcare provider, as compounded or non-FDA-approved versions may pose safety risks. Additionally, these medications should not be used in combination with other GLP-1 receptor agonists or GIP agonists.
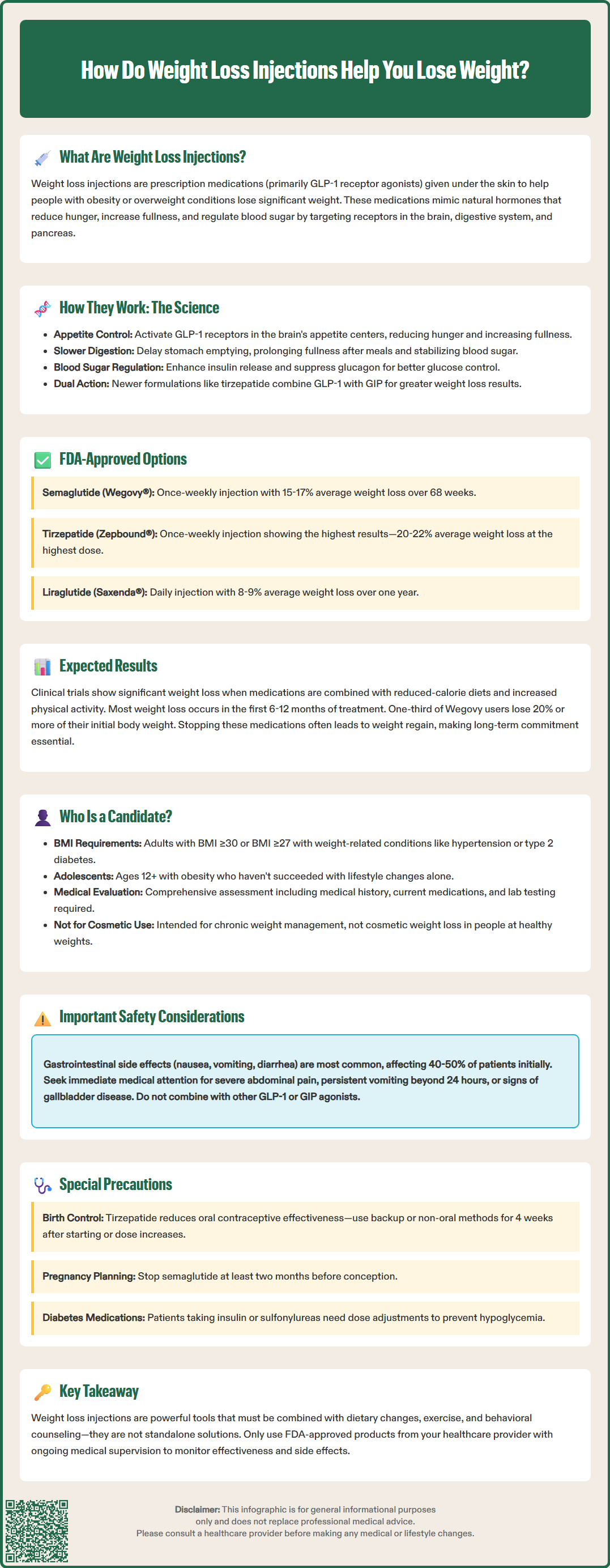
The primary mechanism through which weight loss injections promote weight reduction involves the activation of GLP-1 receptors throughout the body. GLP-1 is an incretin hormone naturally released by intestinal cells in response to food intake. When synthetic GLP-1 receptor agonists bind to these receptors, they trigger several coordinated physiological responses that collectively reduce caloric intake and promote weight loss.
In the brain, these medications act on appetite-regulating centers, particularly in the hypothalamus and brainstem. By activating GLP-1 receptors in these regions, the injections enhance satiety signals and reduce hunger sensations. Patients typically report feeling fuller sooner during meals and experiencing reduced cravings between meals. This neurological effect on appetite regulation represents one of the most significant mechanisms contributing to weight loss, as it helps individuals naturally consume fewer calories without the intense hunger that often undermines traditional calorie-restriction diets.
In the gastrointestinal tract, weight loss injections slow gastric emptying—the rate at which food leaves the stomach and enters the small intestine. This delayed emptying prolongs the sensation of fullness after eating and helps stabilize blood sugar levels by moderating the absorption of nutrients. This effect on gastric emptying can also affect the absorption of oral medications. Notably, tirzepatide (Zepbound) can reduce the effectiveness of oral contraceptives during initiation and dose escalation, requiring backup or non-oral contraception for 4 weeks after starting treatment or increasing the dose.
Additionally, these medications influence pancreatic function by enhancing glucose-dependent insulin secretion and suppressing inappropriate glucagon release. These effects produce significant improvements in glycemic control in individuals with type 2 diabetes or prediabetes, while the effects are more modest in people with normal blood glucose levels.
Some newer formulations combine GLP-1 receptor agonists with other hormones, such as glucose-dependent insulinotropic polypeptide (GIP), creating dual-agonist medications. These combination therapies may offer enhanced weight loss by targeting multiple pathways simultaneously. The synergistic effects of activating both GLP-1 and GIP receptors appear to produce greater reductions in body weight compared to GLP-1 agonists alone, though research continues to elucidate the precise mechanisms underlying this enhanced efficacy.
The FDA has approved several injectable medications specifically for chronic weight management. Semaglutide (marketed as Wegovy®) was approved in 2021 for adults with obesity or overweight with weight-related comorbidities, and in 2022 for adolescents aged 12 and older with obesity. As a once-weekly GLP-1 receptor agonist, clinical trials demonstrated that patients taking semaglutide 2.4 mg weekly lost an average of 15-17% of their body weight over 68 weeks when combined with lifestyle interventions. A lower-dose formulation of semaglutide (Ozempic®) is FDA-approved only for type 2 diabetes management, not for weight loss, though it may be prescribed off-label.
Liraglutide (Saxenda®), approved in 2014 for adults and in 2020 for adolescents aged 12 and older, is administered as a daily subcutaneous injection at a maximum dose of 3.0 mg. As an earlier-generation GLP-1 receptor agonist, liraglutide produces more modest weight loss compared to newer agents, with clinical trials showing average weight reductions of approximately 8-9% over one year. The daily dosing requirement may be less convenient for some patients, though others prefer the flexibility of adjusting doses more frequently.
Tirzepatide (Zepbound®), approved in 2023, functions as a dual GIP/GLP-1 receptor agonist. Clinical trials have demonstrated substantial weight loss outcomes with tirzepatide, with patients losing an average of 20-22% of their body weight at the highest dose (15 mg weekly). This medication is also available as Mounjaro® for type 2 diabetes management, but this formulation is not FDA-approved for weight management.
Setmelanotide (IMCIVREE®) is an injectable medication approved for specific rare genetic forms of obesity caused by defects in the melanocortin-4 receptor pathway.
Key differences among these medications include dosing frequency (daily versus weekly), magnitude of weight loss, side effect profiles, and cost. All require gradual dose escalation over several weeks to minimize gastrointestinal side effects. For patients who do not achieve at least 5% weight loss after 12 weeks on the maintenance dose, healthcare providers may consider discontinuation, as continued treatment may not provide sufficient benefit. Insurance coverage varies significantly, and out-of-pocket costs can be substantial, ranging from several hundred to over one thousand dollars monthly without insurance coverage.
Weight loss outcomes with injectable medications vary considerably based on the specific medication, dose, individual patient factors, and adherence to lifestyle modifications. Clinical trial data provides evidence-based expectations, though individual results may differ. With semaglutide 2.4 mg weekly (Wegovy), participants in the STEP clinical trial program lost an average of 15-17% of their initial body weight over 68 weeks. Approximately one-third of participants achieved weight loss of 20% or more, while about 85% lost at least 5% of their body weight—a threshold associated with meaningful health improvements.
Tirzepatide (Zepbound) has demonstrated substantial weight loss in clinical trials. In the SURMOUNT studies, participants receiving the highest dose (15 mg weekly) lost an average of 20-22% of their body weight over 72 weeks. At the 10 mg dose, average weight loss was approximately 19%, while the 5 mg dose produced about 15% weight reduction. These results represent some of the most significant pharmacologically-induced weight loss outcomes documented in clinical trials to date.
With liraglutide 3.0 mg daily (Saxenda), clinical trials showed average weight loss of 8-9% over one year, with approximately 60% of participants achieving at least 5% weight loss. While more modest than newer agents, this degree of weight reduction still provides clinically meaningful health benefits, including improvements in cardiovascular risk factors, blood pressure, and glycemic control.
It is important to note that these results were achieved in conjunction with reduced-calorie diets and increased physical activity. Weight loss typically occurs gradually, with most reduction happening in the first 6-12 months of treatment. Weight plateaus are common, and some weight regain may occur if medications are discontinued. Patients with type 2 diabetes typically experience somewhat less weight loss compared to those without diabetes. Individual factors such as baseline weight, metabolic health, medication adherence, dietary compliance, and physical activity levels significantly influence outcomes. Patients should maintain realistic expectations and understand that these medications facilitate—but do not replace—lifestyle modifications necessary for sustained weight management.
Weight loss injections are FDA-approved for specific patient populations based on body mass index (BMI) and weight-related health conditions. According to current prescribing guidelines, adult candidates include those with a BMI of 30 kg/m² or greater (classified as obesity) or adults with a BMI of 27 kg/m² or greater (overweight) who have at least one weight-related comorbidity such as hypertension, type 2 diabetes, or dyslipidemia. Additionally, Wegovy and Saxenda are approved for adolescents aged 12 and older with obesity (BMI ≥95th percentile for age and sex).
Ideal candidates are individuals who have attempted to lose weight through lifestyle modifications—including dietary changes and increased physical activity—but have not achieved or maintained clinically significant weight loss. These medications are intended for chronic weight management, not cosmetic weight loss in individuals at healthy weights. Healthcare providers conduct comprehensive evaluations before prescribing, including assessment of medical history, current medications, weight loss history, and motivation for treatment.
Contraindications and precautions must be carefully considered. Weight loss injections are contraindicated in individuals with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2), as GLP-1 receptor agonists have been associated with thyroid C-cell tumors in rodent studies (though no definitive link has been established in humans). Patients with a history of pancreatitis should be evaluated carefully, as these medications may increase pancreatitis risk. Individuals with severe gastrointestinal disease, including gastroparesis, may not tolerate these medications well due to their effects on gastric emptying.
Pregnancy and contraception require special consideration. For semaglutide, discontinuation is recommended at least two months before planned conception due to potential fetal risks. For other agents, discontinuation is advised when pregnancy is recognized. Women using tirzepatide should use backup or non-oral contraception for 4 weeks after starting treatment or increasing the dose, as it can reduce the effectiveness of oral contraceptives. None of these medications are recommended during breastfeeding.
These medications are not indicated for patients with type 1 diabetes for weight management. Comprehensive medical evaluation, including laboratory testing and cardiovascular assessment when indicated, helps identify appropriate candidates and minimize risks.
Weight loss injections are generally well-tolerated, but patients should be informed about potential adverse effects and safety considerations before initiating treatment. The most common side effects are gastrointestinal in nature and include nausea, vomiting, diarrhea, constipation, and abdominal pain. These symptoms typically occur during dose escalation and often diminish over time as the body adjusts to the medication. Nausea affects approximately 40-50% of patients initially but usually resolves within several weeks. Starting at low doses and gradually increasing helps minimize these effects.
To manage gastrointestinal side effects, patients should eat smaller, more frequent meals, avoid high-fat and spicy foods, stay well-hydrated, and eat slowly. If nausea persists or becomes severe, dose reduction or temporary treatment interruption may be necessary. Severe or persistent vomiting can lead to dehydration and electrolyte imbalances, requiring medical attention. Patients should contact their healthcare provider if they cannot tolerate oral fluids or if vomiting persists beyond 24 hours.
Serious but less common adverse effects require awareness and monitoring. Acute pancreatitis has been reported with GLP-1 receptor agonists, though causality remains uncertain. Patients should be instructed to seek immediate medical attention if they experience severe, persistent abdominal pain radiating to the back, as this may indicate pancreatitis. Gallbladder disease, including cholelithiasis (gallstones) and cholecystitis, occurs more frequently with rapid weight loss and has been observed in clinical trials. Symptoms include right upper quadrant pain, nausea, and fever.
Rare cases of intestinal obstruction or ileus have been reported, particularly with semaglutide. Patients should seek urgent care for severe constipation, abdominal distension, or vomiting. FDA labels for these medications include warnings about suicidal behavior and ideation; patients experiencing depression or suicidal thoughts should discontinue the medication and contact their healthcare provider immediately.
Hypoglycemia risk increases when weight loss injections are used in combination with insulin or sulfonylureas, necessitating dose adjustments of these diabetes medications. Injection-site reactions and hypersensitivity, including anaphylaxis, can occur. Patients undergoing surgery should inform their anesthesiologist about these medications, as delayed gastric emptying may affect perioperative management.
Patients should receive counseling about the importance of adherence to follow-up appointments, reporting new or worsening symptoms promptly, and understanding that these medications require long-term commitment. Discontinuation often results in weight regain, highlighting the chronic nature of obesity treatment. Regular monitoring of weight, blood pressure, heart rate, and metabolic parameters helps optimize safety and efficacy throughout treatment.
Most patients experience gradual weight loss over 6-12 months, with the majority of reduction occurring during this period. Clinical trials show meaningful results typically emerge after 12-16 weeks of treatment when combined with lifestyle modifications.
Discontinuing weight loss injections often leads to weight regain, as obesity is a chronic condition requiring ongoing management. Most patients need continued treatment to maintain weight loss, though healthcare providers individualize decisions based on patient circumstances and preferences.
Insurance coverage for weight loss injections varies significantly by plan and provider. Many plans have restrictions or do not cover these medications, resulting in out-of-pocket costs ranging from several hundred to over one thousand dollars monthly without coverage.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.