LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Tirzepatide, marketed as Mounjaro for type 2 diabetes and Zepbound for weight management, has emerged as a powerful dual incretin receptor agonist with effects extending beyond glucose control. While not FDA-approved specifically for cholesterol management, clinical trials reveal that tirzepatide produces meaningful improvements in lipid profiles, particularly triglyceride reduction. Understanding how tirzepatide affects cholesterol levels helps clinicians and patients make informed decisions about metabolic health management, especially for individuals with type 2 diabetes, obesity, or metabolic syndrome seeking comprehensive cardiometabolic benefits.
Quick Answer: Tirzepatide produces modest improvements in cholesterol levels, reducing triglycerides by 15-30%, total cholesterol by 5-10%, and LDL cholesterol by 5-8%, while increasing HDL cholesterol by 5-8%.
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for the treatment of type 2 diabetes mellitus and chronic weight management. Marketed under the brand names Mounjaro (for diabetes) and Zepbound (for weight loss), tirzepatide represents the first dual incretin receptor agonist available in clinical practice.
The medication works through a unique dual mechanism of action. By activating both GIP and GLP-1 receptors, tirzepatide enhances glucose-dependent insulin secretion from pancreatic beta cells, suppresses inappropriate glucagon release, and slows gastric emptying. These combined effects improve glycemic control in patients with type 2 diabetes. Additionally, tirzepatide acts on central appetite regulation pathways in the hypothalamus, leading to reduced caloric intake and significant weight loss.
Tirzepatide is administered as a once-weekly subcutaneous injection, with doses ranging from 2.5 mg to 15 mg depending on the indication and patient tolerance. The medication undergoes dose escalation over several weeks to minimize gastrointestinal side effects, which are the most commonly reported adverse reactions. In clinical trials such as SURPASS-2, tirzepatide demonstrated superior glycemic control and weight reduction compared to semaglutide 1 mg.
Beyond its primary indications, tirzepatide is not approved for type 1 diabetes. The medication carries a boxed warning for thyroid C-cell tumors and has important safety considerations including risks of pancreatitis and gallbladder disease. Secondary findings from clinical trials suggest potential benefits on blood pressure and liver fat content, though these are not FDA-approved indications.
Multiple large-scale clinical trials have evaluated tirzepatide's effects on lipid parameters as secondary endpoints. The SURPASS clinical trial program, which included over 10,000 participants with type 2 diabetes, consistently demonstrated improvements in several lipid markers. Across these studies, tirzepatide treatment was associated with reductions in triglycerides, total cholesterol, and non-HDL cholesterol, along with modest increases in HDL cholesterol (the "good" cholesterol).
In the SURPASS-2 trial, patients receiving tirzepatide 15 mg weekly experienced triglyceride reductions of approximately 20-25% from baseline, with smaller but clinically meaningful decreases in total cholesterol and LDL cholesterol. The SURMOUNT trials, which focused on weight management in individuals with and without diabetes, showed similar lipid improvements. The magnitude of lipid changes appeared dose-dependent and correlated with the degree of weight loss achieved.
Pooled analyses of SURPASS data suggest that tirzepatide reduced triglycerides by approximately 15-30%, total cholesterol by 5-10%, and LDL cholesterol by 5-8% across various doses, with HDL cholesterol increases of approximately 5-8%. These effects may vary by population, dose, and baseline characteristics. Some analyses suggest benefits may occur in patients with and without statin therapy, though more research is needed on specific subgroups.
It is important to note that while these lipid improvements are statistically significant, tirzepatide is not FDA-approved specifically for dyslipidemia treatment. The lipid benefits appear to be secondary effects related to weight loss, improved insulin sensitivity, and direct metabolic actions of the dual incretin pathway. A long-term cardiovascular outcome trial (SURPASS-CVOT) is ongoing to determine whether these lipid changes translate into reduced cardiovascular events.
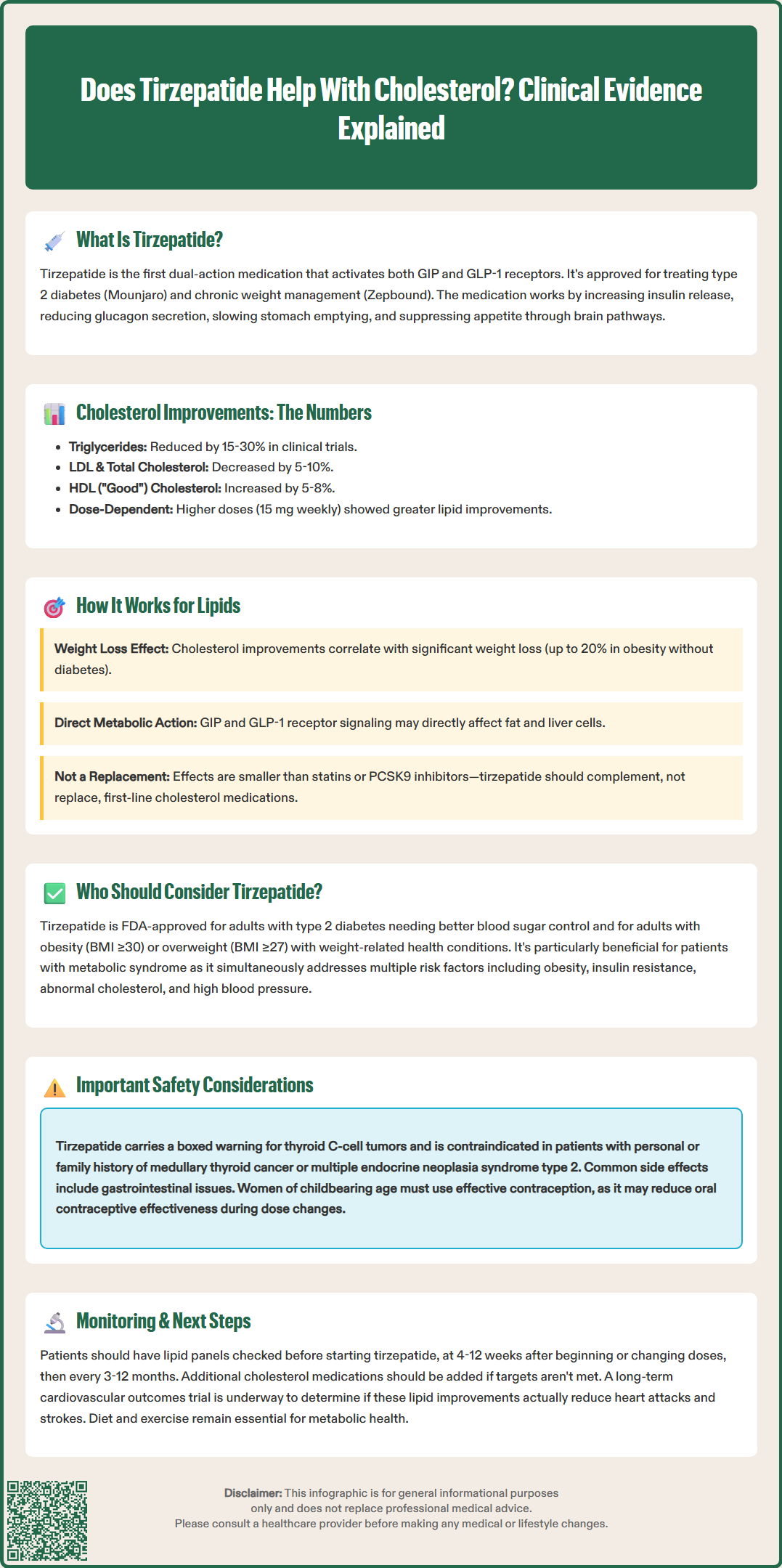
Tirzepatide is FDA-approved for two specific populations: adults with type 2 diabetes mellitus requiring improved glycemic control, and adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. Tirzepatide demonstrates high efficacy for glycemic control and weight reduction in these populations. For patients with established cardiovascular disease, current American Diabetes Association (ADA) guidelines recommend GLP-1 receptor agonists with proven cardiovascular benefit or SGLT2 inhibitors as preferred agents, pending cardiovascular outcomes data for tirzepatide.
Patients with metabolic syndrome—characterized by central obesity, insulin resistance, dyslipidemia, and hypertension—may particularly benefit from tirzepatide's multifaceted effects. The medication addresses multiple components of metabolic syndrome simultaneously, making it an attractive option for individuals with clustering cardiometabolic risk factors. However, prescribing decisions should be individualized based on patient-specific factors, including diabetes status, cardiovascular risk profile, and treatment goals.
Tirzepatide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Patients with severe gastrointestinal disease, particularly severe gastroparesis, may not tolerate tirzepatide well due to its effects on gastric emptying. Additionally, individuals with a history of pancreatitis should be carefully evaluated. Patients should be monitored for signs of pancreatitis (severe abdominal pain) and gallbladder disease (right upper quadrant pain, jaundice) and instructed to seek immediate care if these occur.
Tirzepatide is not recommended during pregnancy, and women of childbearing potential should use effective contraception. Patients should be advised that tirzepatide may reduce the effectiveness of oral contraceptives, particularly during dose initiation or escalation, and backup contraception is recommended for 4 weeks after these changes. When used with insulin or insulin secretagogues, dose adjustments of these medications may be needed to reduce hypoglycemia risk. Patients should maintain adequate hydration, especially those with renal impairment, to reduce acute kidney injury risk associated with gastrointestinal side effects. Cost and insurance coverage represent practical considerations, as tirzepatide is expensive without insurance support.
Based on current clinical evidence, tirzepatide does produce modest improvements in cholesterol levels, though it is not primarily a cholesterol-lowering medication. The most consistent and pronounced effect is on triglyceride reduction, with decreases of 15-30% observed in clinical trials. Total cholesterol and LDL cholesterol (the "bad" cholesterol) typically decrease by 5-10%, while HDL cholesterol shows small increases of 5-8%. These changes are clinically meaningful but generally less dramatic than those achieved with dedicated lipid-lowering therapies such as statins or PCSK9 inhibitors.
The mechanism underlying tirzepatide's lipid effects appears multifactorial. Significant weight loss contributes substantially to lipid improvements, with weight reduction varying by population—up to approximately 20% in people with obesity without diabetes (SURMOUNT-1) and generally less in those with type 2 diabetes. This adipose tissue reduction enhances insulin sensitivity and reduces hepatic lipogenesis. Additionally, tirzepatide may directly influence lipid metabolism through GIP and GLP-1 receptor signaling in adipocytes and hepatocytes, though these mechanisms require further investigation.
For patients with dyslipidemia, tirzepatide should be viewed as a complementary rather than primary intervention. Current guidelines from the American College of Cardiology and American Heart Association continue to recommend statins as first-line therapy for LDL cholesterol reduction in patients with elevated cardiovascular risk. For patients with severe hypertriglyceridemia (≥500 mg/dL), dedicated triglyceride-lowering therapy and specialist referral should be considered regardless of tirzepatide use.
Patients considering tirzepatide for metabolic health should undergo appropriate lipid assessment before and during treatment. Monitoring should include a lipid panel (fasting or nonfasting) at baseline and 4-12 weeks after initiation or dose changes, then periodically every 3-12 months per ACC/AHA guidance. If lipid goals are not achieved with tirzepatide alone, additional lipid-lowering therapies should be considered according to established guidelines. Importantly, tirzepatide is not FDA-approved for cardiovascular risk reduction, and cardiovascular outcome data are pending. Lifestyle modifications including diet and exercise remain foundational to optimal metabolic health.
No, tirzepatide should not replace statins for cholesterol management. Statins remain first-line therapy for LDL cholesterol reduction per ACC/AHA guidelines, and tirzepatide is not FDA-approved for dyslipidemia treatment.
Lipid improvements typically become apparent within 4-12 weeks of starting tirzepatide or after dose changes. Monitoring with a lipid panel is recommended during this timeframe and then periodically every 3-12 months.
Patients with metabolic syndrome, type 2 diabetes, or obesity who have elevated triglycerides and multiple cardiometabolic risk factors may benefit most. However, prescribing decisions should be individualized based on FDA-approved indications and patient-specific factors.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.