LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Many patients starting tirzepatide (Mounjaro, Zepbound) wonder about its effects on fluid balance and whether it might cause or alleviate water retention. Understanding how this dual GIP/GLP-1 receptor agonist influences the body's fluid homeostasis is important for managing expectations and recognizing potential concerns. Unlike some diabetes medications that directly promote edema, tirzepatide does not typically cause water retention as a characteristic side effect. This article examines the relationship between tirzepatide and fluid balance, explores how the medication works, and provides guidance on managing water retention concerns during treatment.
Quick Answer: Tirzepatide does not typically cause water retention and is not associated with edema as a characteristic side effect, unlike some other diabetes medications.
Water retention, medically termed edema, occurs when excess fluid accumulates in the body's tissues, most commonly in the legs, ankles, feet, and hands. This condition manifests as swelling, puffiness, and sometimes discomfort or a feeling of heaviness in affected areas. Understanding the underlying causes is essential for appropriate management and determining whether medications like tirzepatide might influence fluid balance.
Several medical conditions contribute to water retention. Cardiovascular disease , particularly congestive heart failure, impairs the heart's ability to pump blood efficiently, leading to fluid backup in peripheral tissues. Kidney disease reduces the body's capacity to eliminate excess sodium and water, while liver disease can decrease protein production, affecting fluid distribution. Venous insufficiency, where leg veins struggle to return blood to the heart, commonly causes lower extremity swelling. Hormonal fluctuations, certain medications, and dietary factors also play significant roles.
In patients with type 2 diabetes, water retention may occur due to multiple factors. Poor glycemic control can affect kidney function over time, while some diabetes medications—including insulin (which can cause edema regardless of formulation, particularly after initiation or dose intensification) and thiazolidinediones—are known to cause fluid retention. Additionally, diabetic patients often have comorbid conditions like hypertension and heart disease that independently contribute to edema. The relationship between diabetes medications and fluid balance requires careful clinical consideration, as some agents may worsen retention while others have neutral or potentially beneficial effects on fluid homeostasis.
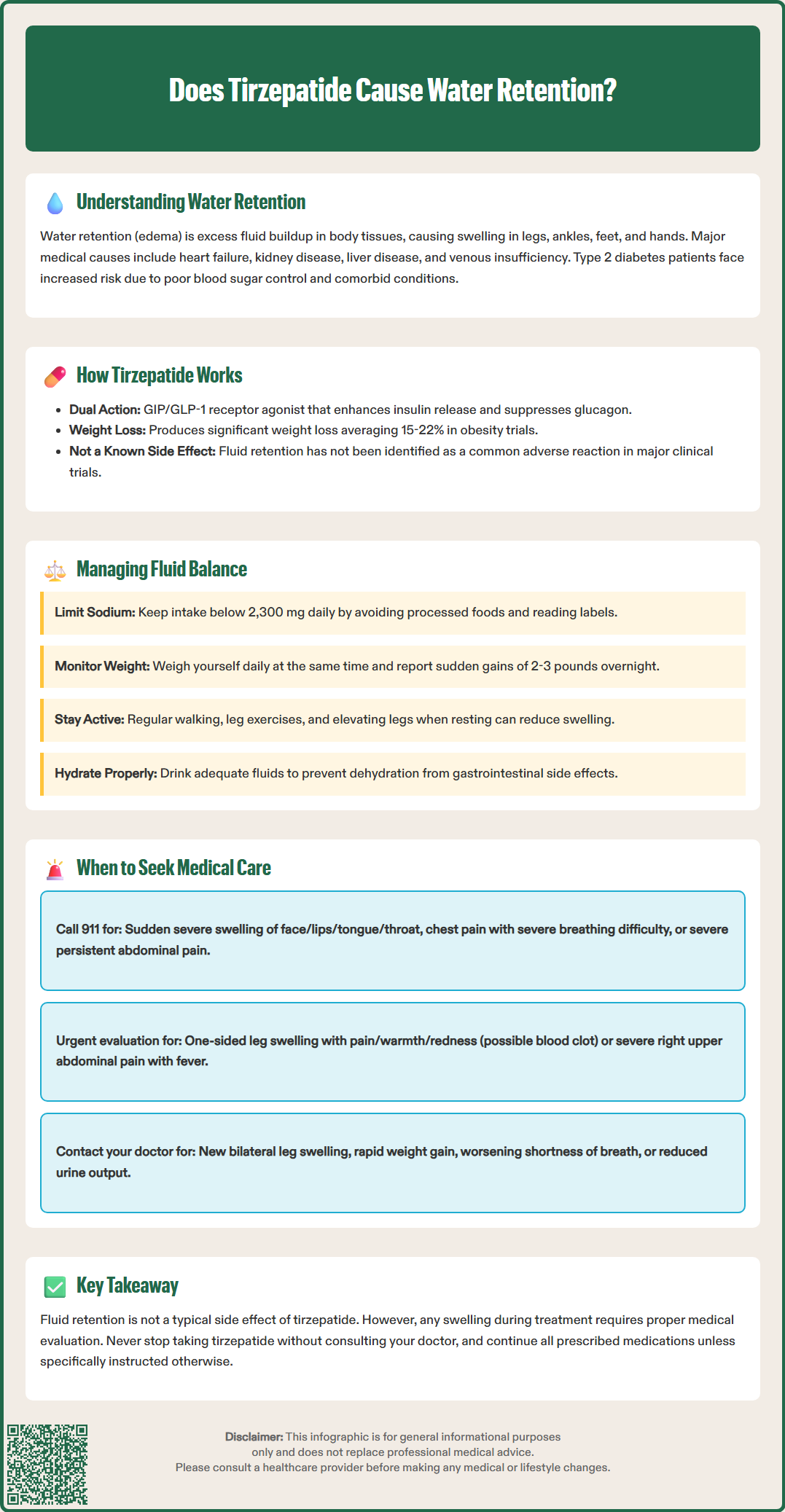
Tirzepatide (Mounjaro, Zepbound) represents a novel class of medication known as a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. Approved by the FDA for type 2 diabetes management and chronic weight management, tirzepatide works through multiple complementary mechanisms that extend beyond simple glucose control.
The medication's primary mechanisms of action include:
Enhanced insulin secretion: Stimulates pancreatic beta cells to release insulin in response to elevated blood glucose
Reduced glucagon release: Suppresses inappropriate glucagon secretion from pancreatic alpha cells
Delayed gastric emptying: Slows food transit from the stomach, promoting satiety (though this effect may diminish over time)
Central appetite regulation: Acts on brain centers to reduce hunger and food intake
Improved metabolic function: Weight loss from tirzepatide contributes to improved insulin sensitivity
Regarding water retention specifically, fluid retention is not listed as a characteristic adverse effect of tirzepatide in FDA prescribing information or major clinical trials. Unlike thiazolidinediones (such as pioglitazone), which activate peroxisome proliferator-activated receptors and directly promote sodium and water retention, tirzepatide's receptor targets do not have this established effect. Clinical trial data from the SURPASS program (for type 2 diabetes) and SURMOUNT program (for obesity) have not identified edema as a common adverse reaction.
Tirzepatide may indirectly affect fluid balance through weight loss—averaging approximately 15-22% in obesity trials without diabetes and more modest reductions in type 2 diabetes trials. This weight reduction may theoretically reduce mechanical stress on the venous system. However, it's important to note that definitive cardiovascular and renal outcome data for tirzepatide are still being investigated, and established clinical benefits on fluid homeostasis have not yet been determined.
While tirzepatide is not typically associated with water retention, patients starting this medication should maintain awareness of fluid balance as part of comprehensive diabetes and weight management. Several practical strategies can help optimize fluid homeostasis during treatment.
Dietary sodium management remains fundamental to controlling water retention. The American Diabetes Association generally recommends limiting sodium intake to less than 2,300 mg daily for most adults with diabetes. More stringent sodium restriction (such as 1,500 mg daily) may be individualized for those with hypertension, heart failure, or existing fluid retention issues, based on clinical guidelines from cardiovascular societies. Reading nutrition labels, minimizing processed foods, and avoiding added salt can significantly impact fluid balance.
Monitoring and documentation help identify concerning patterns early. Patients should:
Weigh themselves at the same time daily (particularly important for those with or at risk for heart failure), reporting sudden gains of 2-3 pounds overnight or 5 pounds within a week
Note any new or worsening swelling in the legs, ankles, or abdomen
Track blood pressure regularly, as hypertension often accompanies fluid retention
Maintain a symptom diary including shortness of breath or reduced exercise tolerance
Lifestyle modifications support healthy fluid balance. Regular physical activity, particularly leg exercises and walking, promotes venous return and reduces lower extremity swelling. Elevating the legs when resting, wearing compression stockings if recommended by a healthcare provider, and staying adequately hydrated all contribute to optimal fluid management.
Maintaining hydration is particularly important with tirzepatide, as its gastrointestinal side effects (nausea, vomiting, diarrhea) can lead to dehydration. Patients should drink adequate fluids and contact their healthcare provider if experiencing persistent GI symptoms, dizziness, or reduced urine output, as these may indicate dehydration and potential acute kidney injury.
Patients should continue all prescribed medications for comorbid conditions unless specifically instructed otherwise. Diuretics, ACE inhibitors, or other cardiovascular medications should not be discontinued without medical guidance, even if weight loss from tirzepatide improves overall health status. Coordination between prescribers ensures comprehensive management of fluid balance alongside diabetes and weight control.
While tirzepatide is not typically associated with water retention, patients should remain vigilant for signs of fluid accumulation that may indicate underlying medical conditions requiring evaluation. Distinguishing between minor, self-limited swelling and clinically significant edema is essential for timely intervention.
Seek immediate medical attention (call 911 or go to the emergency department) if you experience:
Sudden, severe swelling of the face, lips, tongue, or throat, which may indicate a serious allergic reaction
Chest pain, severe shortness of breath, or inability to lie flat due to breathing difficulty
Severe, persistent abdominal pain (often radiating to the back) with or without vomiting, which could suggest pancreatitis (a known rare risk with GLP-1 receptor agonists)
Seek urgent medical evaluation for:
One-sided leg swelling with pain, warmth, and redness, potentially indicating deep vein thrombosis
Severe right upper abdominal pain, fever, or yellowing of the skin/eyes, which may suggest gallbladder disease (a risk with GLP-1 receptor agonists and rapid weight loss)
Contact your healthcare provider within 24-48 hours for:
New or worsening bilateral leg swelling that doesn't improve with elevation
Rapid weight gain (more than 2-3 pounds overnight or 5 pounds in one week)
Increasing shortness of breath with exertion or when lying down
Reduced urine output or changes in urine color
Persistent swelling around the eyes or in the hands
Swelling accompanied by fatigue, confusion, or other systemic symptoms
Your healthcare provider will conduct a thorough evaluation to determine the cause of fluid retention. This typically includes physical examination, review of all current medications, assessment of kidney and liver function through blood tests, and possibly cardiac evaluation with electrocardiogram or echocardiography. The provider may adjust other medications, recommend dietary modifications, or prescribe diuretics if appropriate.
It is important to note that while edema is not a typical side effect of tirzepatide, any swelling that develops during therapy should be evaluated promptly. Potential causes include hypersensitivity reactions, injection site reactions, or underlying cardiovascular, renal, or hepatic conditions. Never discontinue tirzepatide or any prescribed medication without consulting your healthcare provider, as abrupt cessation may adversely affect glycemic control or weight management progress.
Tirzepatide is not typically associated with water retention or edema as a side effect. Unlike some diabetes medications such as thiazolidinediones, tirzepatide's mechanism of action does not directly promote fluid accumulation, and clinical trials have not identified edema as a common adverse reaction.
Contact your healthcare provider if you experience new or worsening swelling, rapid weight gain, or reduced urine output while taking tirzepatide. Seek immediate medical attention for sudden facial swelling, severe shortness of breath, or one-sided leg swelling with pain, as these may indicate serious conditions requiring urgent evaluation.
Tirzepatide works as a dual GIP/GLP-1 receptor agonist and does not activate the pathways that promote sodium and water retention. In contrast, thiazolidinediones and insulin can cause edema through different mechanisms, making tirzepatide a preferable option for patients concerned about fluid retention.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.