LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Semaglutide has been studied for over 14 years, with formal clinical research beginning in the early 2010s. This glucagon-like peptide-1 receptor agonist (GLP-1 RA), developed by Novo Nordisk, has undergone extensive investigation through multiple clinical trial programs. The SUSTAIN trials for type 2 diabetes began enrolling patients in 2012, followed by the STEP trials for weight management. Since its first FDA approval in December 2017, semaglutide has accumulated approximately 7 years of real-world post-marketing data. This comprehensive research timeline provides substantial evidence regarding efficacy, safety, and cardiovascular benefits across diverse patient populations, making semaglutide one of the most thoroughly studied medications in its class.
Quick Answer: Semaglutide has been studied for over 14 years, with clinical trials beginning in the early 2010s and approximately 7 years of real-world post-marketing experience since FDA approval in December 2017.
Semaglutide has been under scientific investigation for over a decade, with formal research beginning in the early 2010s. Developed by Novo Nordisk as a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide emerged from efforts to create longer-acting diabetes medications with improved glycemic control and potential cardiovascular benefits.
The compound's preclinical development phase included animal studies examining its pharmacokinetic properties and mechanism of action. Semaglutide was specifically engineered with structural modifications to extend its half-life to approximately one week, enabling once-weekly subcutaneous administration. These modifications include an Ala8→Aib amino acid substitution and attachment of a C18 fatty diacid side chain at Lys26 via a linker that promotes albumin binding, significantly prolonging circulation time compared to native GLP-1.
The first human clinical trials began in the early 2010s, marking the beginning of a comprehensive clinical development program. The SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) trial series began enrolling patients in 2012, ultimately encompassing multiple phase 3 trials with thousands of participants worldwide. These pivotal studies evaluated semaglutide's efficacy and safety across diverse patient populations, including those with varying diabetes duration, renal impairment, and cardiovascular risk profiles.
By 2024, semaglutide has accumulated over 14 years of human clinical trial data and approximately 7 years of real-world post-marketing experience since its initial regulatory approval in December 2017. This extensive research timeline provides substantial evidence regarding both short-term efficacy and longer-term safety considerations, though ongoing studies continue to expand our understanding of this medication's full clinical profile.
The FDA approval pathway for semaglutide followed a rigorous, phased approach spanning several years. Phase 1 trials established basic safety, tolerability, and pharmacokinetic parameters in healthy volunteers and small groups of patients with type 2 diabetes. These early studies confirmed the once-weekly dosing feasibility and identified the gastrointestinal side effects—primarily nausea, vomiting, and diarrhea—that would become characteristic of GLP-1 receptor agonist therapy.
Phase 2 trials focused on dose-ranging studies to identify optimal therapeutic doses. Researchers evaluated 0.5 mg, 1.0 mg, and higher doses, assessing glycemic efficacy measured by HbA1c reduction alongside safety parameters. These trials demonstrated dose-dependent improvements in glycemic control and notable weight reduction, prompting further investigation at the 0.5 mg and 1.0 mg maintenance doses.
The SUSTAIN phase 3 program provided the pivotal efficacy and safety data supporting regulatory submissions. Key trials included SUSTAIN 6, a cardiovascular outcomes trial demonstrating significant reduction in major adverse cardiovascular events (MACE) compared to placebo (HR 0.74; median follow-up 2.1 years), which proved instrumental in the approval process. The FDA granted approval for Ozempic (semaglutide injection) for type 2 diabetes in December 2017, with initial doses of 0.5 mg and 1.0 mg weekly. A 2 mg dose was later approved in 2022.
Subsequent approvals expanded semaglutide's indications and formulations. In September 2019, the FDA approved Rybelsus, the first oral GLP-1 receptor agonist, available in 3 mg (starter dose), 7 mg, and 14 mg daily tablets. In June 2021, the FDA approved Wegovy (semaglutide 2.4 mg) specifically for chronic weight management in adults with obesity or overweight with weight-related comorbidities. This approval was based on the STEP (Semaglutide Treatment Effect in People with obesity) clinical trial program, which enrolled over 4,500 participants.
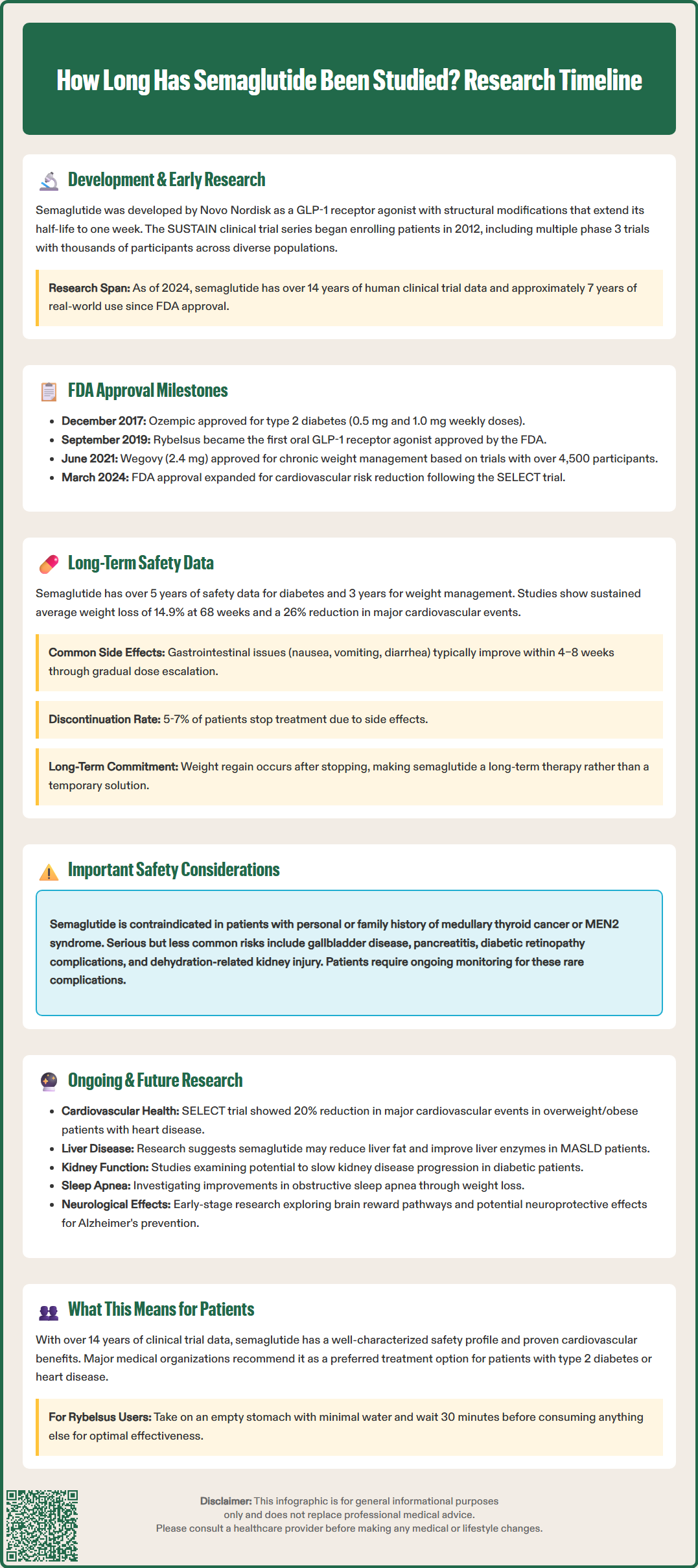
Long-term data on semaglutide now extends beyond five years for diabetes indications and approximately three years for weight management, with ongoing extension studies continuing to accumulate evidence. The SUSTAIN 6 cardiovascular outcomes trial, which followed patients for a median of 2.1 years, demonstrated a 26% reduction in the primary composite endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke compared to placebo. This established semaglutide's cardiovascular safety profile and suggested potential protective benefits.
The STEP program for obesity management has provided crucial long-term weight loss data. STEP 1, the largest trial with 1,961 participants, demonstrated that after 68 weeks of treatment, patients achieved an average weight loss of 14.9% compared to 2.4% with placebo. The STEP 5 trial provided 104-week data showing sustained efficacy. Extension studies have shown that weight loss is generally maintained with continued therapy, though significant weight regain occurs upon discontinuation—an important consideration for long-term treatment planning.
Safety signals identified through extended follow-up include:
Gastrointestinal effects: Nausea, vomiting, and diarrhea remain the most common adverse events, typically diminishing over time but causing discontinuation in approximately 5–7% of patients
Gallbladder disease: Increased incidence of cholelithiasis and cholecystitis, possibly associated with weight loss, though the exact mechanism remains under investigation
Diabetic retinopathy: Risk of complications, particularly with rapid glycemic improvement in patients with pre-existing retinopathy
Acute kidney injury: Risk associated with severe gastrointestinal adverse events leading to dehydration
Pancreatitis: Rare cases reported, though causality remains uncertain; the FDA label includes a warning based on class effects of GLP-1 receptor agonists
Thyroid C-cell tumors: Observed in rodent studies but no confirmed cases in humans; contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2
Post-marketing surveillance through the FDA Adverse Event Reporting System (FAERS) and international pharmacovigilance databases continues to monitor for rare or delayed adverse effects. Real-world evidence studies, now encompassing hundreds of thousands of patients, generally confirm the clinical trial safety profile while providing insights into effectiveness in more diverse populations and clinical settings.
Current research on semaglutide extends well beyond its approved indications, with numerous ongoing trials investigating potential applications in various disease states. The SELECT trial (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity), completed in 2023 with over 17,600 participants, demonstrated a 20% reduction in major adverse cardiovascular events in patients with established cardiovascular disease and overweight or obesity but without diabetes. This landmark study led to FDA approval expansion in March 2024 for Wegovy (semaglutide 2.4 mg) to reduce the risk of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in adults with cardiovascular disease and either obesity or overweight.
Several active research areas include:
Metabolic dysfunction-associated steatotic liver disease (MASLD): Multiple trials are evaluating semaglutide's effects on liver fat content and biomarkers, with preliminary data suggesting potential benefits in reducing hepatic steatosis and improving liver enzyme profiles
Chronic kidney disease: Studies examining renal outcomes in patients with type 2 diabetes and established kidney disease, building on secondary analyses from SUSTAIN 6 showing reduced progression of diabetic nephropathy
Obstructive sleep apnea: Ongoing trials investigating whether weight loss achieved with semaglutide improves sleep apnea severity and related cardiovascular outcomes
Addiction and substance use disorders: Early-phase research exploring GLP-1 receptor agonists' potential effects on reward pathways and addictive behaviors, though this remains highly preliminary
Alzheimer's disease and cognitive function: Preclinical evidence suggesting neuroprotective properties has prompted clinical trials examining cognitive outcomes in at-risk populations
Researchers are also investigating combination therapies, such as cagrilintide plus semaglutide (CagriSema), which is currently under development. Pharmaceutical development continues with next-generation formulations, including higher-dose preparations and alternative delivery systems aimed at improving convenience and adherence.
The extensive research timeline for semaglutide provides patients and clinicians with a robust evidence base spanning diverse populations and clinical scenarios. With over 14 years of clinical trial data and 7 years of real-world use, semaglutide represents one of the more thoroughly studied medications in its class, though it's important to recognize that very long-term data (beyond 5–7 years of continuous use) remains limited.
For patients considering semaglutide therapy, this research timeline offers several practical implications. The well-characterized safety profile means that common adverse effects are predictable and manageable. Gastrointestinal symptoms, while frequent, typically improve within 4–8 weeks as the body adjusts to therapy. Gradual dose escalation, as recommended in FDA labeling, helps minimize these effects. Patients should be counseled that these symptoms are expected and generally not indicative of serious pathology, though persistent or severe symptoms warrant medical evaluation.
The cardiovascular benefits demonstrated in long-term trials are particularly relevant for patients with type 2 diabetes or established heart disease. The American Diabetes Association (ADA) and American College of Cardiology (ACC) now recommend GLP-1 receptor agonists with proven cardiovascular benefit, including semaglutide, as preferred agents for patients with diabetes and atherosclerotic cardiovascular disease. This evidence-based recommendation reflects the maturity of the research supporting these medications.
However, patients should understand that ongoing monitoring remains essential. Healthcare providers should assess for:
Persistent gastrointestinal symptoms that may indicate gallbladder disease
Signs of pancreatitis (severe abdominal pain radiating to the back)
Changes in vision or symptoms of diabetic retinopathy progression, especially in patients with pre-existing retinopathy
Signs of dehydration or acute kidney injury with severe gastrointestinal symptoms
Hypoglycemia when used with insulin or sulfonylureas (which may require dose reduction of these medications)
Symptoms related to the boxed warning (thyroid tumors): neck mass, hoarseness, dysphagia, or dyspnea
Patients should be aware that semaglutide is contraindicated during pregnancy and lactation, and women of childbearing potential should stop treatment approximately 2 months before a planned pregnancy.
For patients taking Rybelsus (oral semaglutide), proper administration is crucial: take on an empty stomach with no more than 4 ounces of plain water, then wait at least 30 minutes before eating, drinking, or taking other medications.
The research timeline also highlights an important limitation: long-term data on weight maintenance after discontinuation is still emerging. Current evidence suggests that most patients regain significant weight within one year of stopping semaglutide, indicating that this is likely a long-term or indefinite therapy for obesity management rather than a short-term intervention. Patients should discuss realistic expectations and treatment duration with their healthcare providers before initiating therapy.
Finally, while the expanding research into additional indications is promising, patients should be aware that off-label use for conditions like sleep apnea or cognitive decline lacks the rigorous evidence supporting approved indications. Such uses should only be considered within the context of clinical trials or after careful discussion of the limited evidence and potential risks with a qualified healthcare provider.
Clinical trials for semaglutide began in the early 2010s, with the SUSTAIN phase 3 trial program enrolling patients starting in 2012. The first FDA approval for Ozempic (semaglutide injection) for type 2 diabetes was granted in December 2017.
Long-term data on semaglutide now extends beyond 5 years for diabetes indications and approximately 3 years for weight management. The medication has accumulated over 14 years of clinical trial data and approximately 7 years of real-world post-marketing experience.
The SUSTAIN 6 trial demonstrated a 26% reduction in major adverse cardiovascular events in patients with type 2 diabetes. The SELECT trial, completed in 2023 with over 17,600 participants, showed a 20% reduction in cardiovascular events in patients with established cardiovascular disease and overweight or obesity without diabetes.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.