LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Tirzepatide and food noise has become a topic of significant interest among individuals seeking weight management solutions. Tirzepatide, a dual GIP/GLP-1 receptor agonist approved by the FDA for type 2 diabetes and chronic weight management, appears to reduce persistent food-related thoughts that many describe as "food noise." While not a formal medical term, food noise refers to intrusive, constant thoughts about eating that can interfere with daily life. Clinical trials demonstrate substantial weight loss with tirzepatide, and many patients report decreased food preoccupation, though individual experiences vary considerably.
Quick Answer: Tirzepatide, a dual GIP/GLP-1 receptor agonist, appears to reduce persistent food-related thoughts ("food noise") through appetite regulation mechanisms, though individual responses vary considerably.
The phenomenon extends beyond normal hunger signals. Individuals experiencing food noise often describe an overwhelming mental chatter about food choices, portion sizes, and eating opportunities that persists even after consuming adequate calories. This cognitive burden can lead to frequent snacking, difficulty adhering to meal plans, and emotional distress. Some research suggests these experiences may be related to appetite-regulating hormones and reward pathways, though the exact mechanisms remain under investigation.
The impact on eating behavior can be substantial. These persistent food thoughts may drive increased caloric intake through frequent eating episodes, larger portion sizes, and selection of highly palatable, energy-dense foods. Many individuals report that this constant mental preoccupation interferes with work productivity, social interactions, and overall quality of life. The psychological toll includes feelings of guilt, shame, and frustration.
Understanding these food-related thoughts as potentially having physiological components rather than simply reflecting lack of willpower represents an important perspective in obesity medicine. For some individuals, these experiences may overlap with diagnosable conditions like binge-eating disorder, which requires specialized evaluation and treatment. Healthcare providers may use validated tools like the Binge Eating Disorder Screener-7 (BEDS-7) to assess for eating disorders and refer to appropriate specialists when indicated.
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (as Mounjaro) and chronic weight management (as Zepbound). Its unique dual-agonist mechanism distinguishes it from single-receptor GLP-1 agonists and contributes to its effects on appetite regulation.
According to the FDA prescribing information, tirzepatide works through GIP and GLP-1 receptor activation, which affects energy intake and expenditure while slowing gastric emptying. The medication appears to influence areas involved in appetite control, including the hypothalamus and brainstem, where GLP-1 receptors help regulate hunger signals and promote satiety after meals. Tirzepatide may also affect the reward aspects of eating—the pleasure-seeking component that often underlies food cravings. This dual action potentially addresses both homeostatic hunger (eating for energy needs) and hedonic hunger (eating for pleasure or reward).
The GIP component of tirzepatide adds metabolic benefits that may complement the GLP-1 effects. GIP receptors are found in adipose tissue and the central nervous system, where they influence energy metabolism. The synergistic effect of dual receptor activation appears to produce substantial weight loss and appetite reduction, though the complete mechanism of action is not fully characterized.
Pharmacologically, tirzepatide slows gastric emptying, which prolongs the sensation of fullness after eating, though this effect tends to diminish somewhat over time with continued treatment. Patients frequently report that food becomes "less interesting" or that they can "take it or leave it" rather than experiencing constant cravings. However, it's important to note that changes in subjective food thoughts have not been directly measured as primary endpoints in clinical trials.
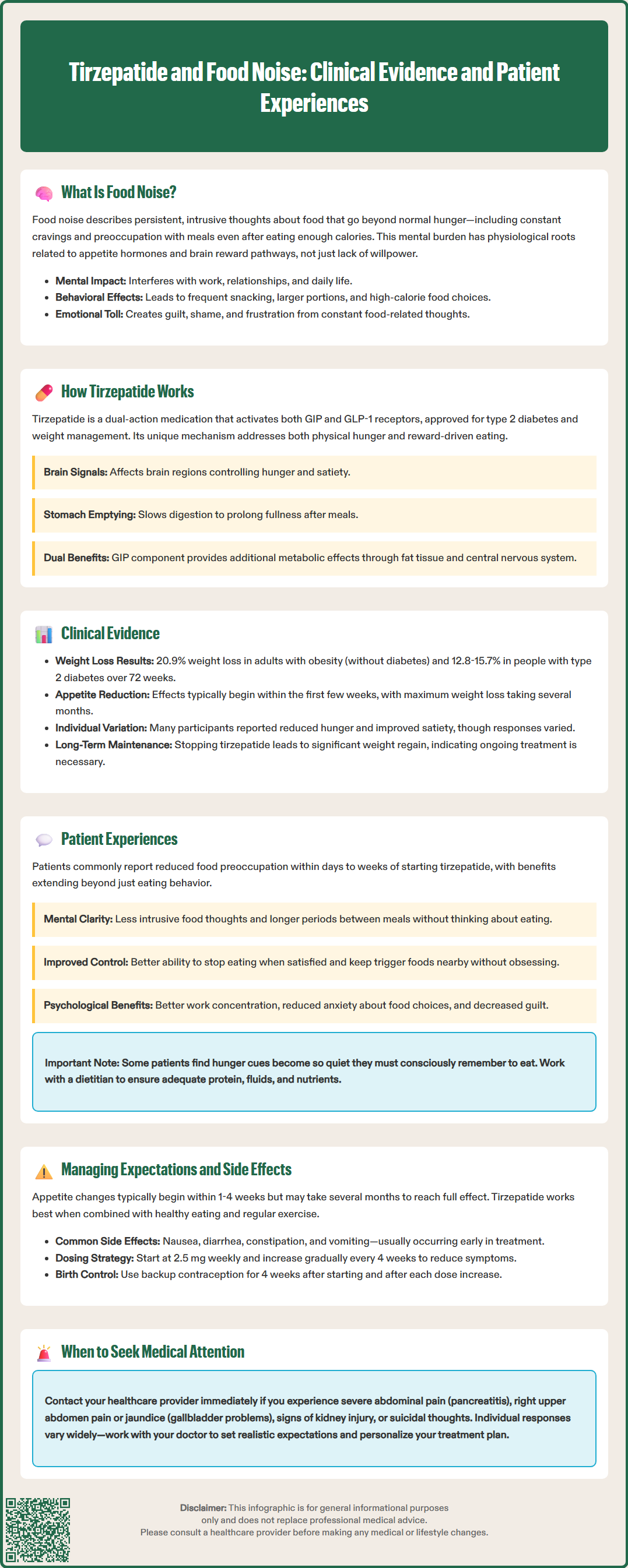
The SURMOUNT clinical trial program provides robust evidence for tirzepatide's effects on weight management. In the SURMOUNT-1 trial (Jastreboff et al., NEJM 2022), adults with obesity (without diabetes) receiving tirzepatide 15 mg weekly achieved a mean weight loss of 20.9% over 72 weeks, compared to 3.1% with placebo. In SURMOUNT-2 (Frías et al., NEJM 2023), participants with type 2 diabetes and obesity achieved mean weight reductions of 12.8% to 15.7% depending on dose.
While these trials demonstrated significant weight loss, they did not specifically measure "food noise" as a primary endpoint. Secondary outcomes included some patient-reported measures related to appetite and eating behaviors. Many participants receiving tirzepatide reported improvements in hunger and satiety, though individual responses varied considerably. It's worth noting that no head-to-head randomized controlled trials have directly compared tirzepatide to other obesity medications like semaglutide 2.4 mg in the obesity setting.
The durability of weight loss effects has been examined in the SURMOUNT-4 trial (Rubino et al., JAMA 2023), which demonstrated that discontinuation of tirzepatide after successful weight loss led to significant weight regain, suggesting ongoing treatment may be necessary for sustained benefits. Many patients report reduced hunger and food thoughts within the first few weeks of therapy, though the maximum effect on weight typically takes months to develop.
Real-world evidence continues to accumulate regarding appetite effects. Factors influencing response may include baseline eating behaviors, psychological relationship with food, concurrent medications, and adherence to lifestyle modifications. The durability of appetite suppression over extended periods (beyond two years) remains an area of ongoing investigation, with long-term extension studies underway.
Patient testimonials and qualitative research reveal common themes regarding food-related thought changes with tirzepatide therapy. These anecdotal reports should not be considered predictive of individual responses, as experiences vary widely. Many individuals describe a noticeable reduction in food preoccupation, reporting that constant food thoughts that previously dominated their mental landscape become less intrusive. This subjective experience often begins within days to weeks of initiating treatment or increasing the dose.
Common descriptions include the ability to go longer between meals without thinking about food, reduced interest in previously tempting foods, and increased capacity to stop eating when satisfied rather than when uncomfortably full. Some patients report surprise at their changed relationship with food, noting they can keep trigger foods in the house without obsessing over them or can attend social events with less anxiety about overeating.
The psychological relief accompanying these changes can extend beyond eating behavior. Some patients report improved concentration at work, reduced anxiety related to food choices, and decreased guilt previously associated with eating. However, this shift can be disconcerting initially, particularly if food has served emotional or social functions beyond nutrition.
Not all experiences are uniformly positive. Some patients report that while food thoughts decrease, they must consciously remember to eat adequate nutrition, as natural hunger cues become very quiet. This may require structured meal planning and monitoring to ensure adequate protein, fluid, and nutrient intake. Working with a registered dietitian can be beneficial in these cases. Others note that the effect diminishes somewhat over time. A subset of patients experiences minimal change in food thoughts despite weight loss. Individual variation in response underscores the importance of personalized treatment approaches and realistic expectation-setting.
Setting realistic expectations is essential for successful tirzepatide therapy. While many patients experience significant appetite changes, the timeline and magnitude of effect vary considerably. Most individuals notice some appetite changes within 1-4 weeks of starting treatment or dose escalation, but maximal effects may take several months to manifest. Patients should understand that tirzepatide is an adjunct to—not a replacement for—lifestyle modifications including balanced nutrition and regular physical activity.
The most common adverse effects are gastrointestinal: nausea, diarrhea, constipation, vomiting, and abdominal discomfort. These symptoms typically occur early in treatment or after dose increases and often diminish over time. Starting at a low dose (2.5 mg weekly) and gradually titrating upward every 4 weeks helps minimize gastrointestinal side effects. The FDA-approved titration schedule increases by 2.5 mg increments up to the maximum 15 mg weekly dose. Patients should be advised to eat smaller, more frequent meals, avoid high-fat foods, and stay well-hydrated during the adjustment period.
More serious but less common adverse effects require vigilance. Tirzepatide carries warnings for thyroid C-cell tumors (based on rodent studies), acute pancreatitis, gallbladder disease, acute kidney injury, diabetic retinopathy complications in patients with diabetes, and hypoglycemia when used with insulin or sulfonylureas. Zepbound (tirzepatide for weight management) also carries a warning about suicidal behavior and ideation; patients should be monitored for depression or suicidal thoughts. Patients should seek immediate medical attention for severe abdominal pain, persistent vomiting, right upper quadrant pain, yellowing of skin/eyes, or signs of allergic reaction.
Additional important considerations include:
Tirzepatide is not recommended during pregnancy or breastfeeding
The medication may decrease the effectiveness of oral contraceptives; additional contraceptive methods are recommended for 4 weeks after initiation and after each dose increase
Tirzepatide is not recommended in patients with severe gastrointestinal disease, including severe gastroparesis
Patients should inform all healthcare providers, including surgical teams, about tirzepatide use
Treatment is likely long-term, as weight regain typically occurs upon discontinuation
Patients should be monitored for adequate nutrition and hydration, particularly if appetite suppression is pronounced. Working with a registered dietitian can help ensure nutritional needs are met despite reduced hunger.
Most patients notice some appetite changes within 1-4 weeks of starting tirzepatide or after dose increases, though maximal effects may take several months to develop. Individual responses vary considerably, and some patients experience minimal changes in food thoughts despite weight loss.
No head-to-head randomized controlled trials have directly compared tirzepatide to semaglutide 2.4 mg for obesity treatment or appetite effects. Both medications work through GLP-1 receptor activation, though tirzepatide also activates GIP receptors, which may contribute to its metabolic effects.
If appetite suppression becomes excessive, work with your healthcare provider and consider consulting a registered dietitian to ensure adequate nutrition, protein, and fluid intake. You may need structured meal planning and monitoring to meet nutritional needs despite reduced hunger cues.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.