LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Finding what looks like an intact metformin tablet in your stool can be concerning, but this is typically a normal occurrence with extended-release formulations. Undigested pills in stool metformin situations usually involve empty tablet shells designed to control medication release over time. These specialized delivery systems use indigestible outer casings that remain structurally intact after the active drug has been absorbed. Understanding why this happens, when it's expected, and when to seek medical advice can help you confidently manage your diabetes treatment and ensure your medication is working effectively.
Quick Answer: Undigested metformin pills in stool are typically empty tablet shells from extended-release formulations, indicating the delivery system worked as designed rather than medication failure.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Finding what appears to be an intact metformin tablet in your stool can be alarming, but this occurrence is typically related to the formulation design rather than a medication failure. Metformin, particularly extended-release (ER) formulations, uses specialized delivery systems that control how the active ingredient is released in your gastrointestinal tract.
Extended-release metformin tablets employ matrix or osmotic pump technologies that allow the medication to dissolve slowly over several hours. The outer shell or matrix of these tablets is often made from indigestible materials such as cellulose polymers or other pharmaceutical excipients. As the active metformin is gradually released and absorbed through the intestinal wall, the empty shell continues through your digestive system.
This ghost tablet phenomenon is most commonly associated with osmotic-controlled release oral delivery systems (like Fortamet) and hydrophilic matrix systems (like Glucophage XR), where the tablet shell or matrix remains structurally intact even after the medication has been delivered. According to FDA-approved labeling for several metformin ER products, patients may occasionally notice tablet remnants in their stool, and this is expected and does not indicate treatment failure.
Immediate-release metformin formulations typically dissolve completely and are less likely to produce visible tablet remnants. However, factors such as rapid gastrointestinal transit time or certain digestive conditions may occasionally result in incomplete dissolution even with standard formulations.
Extended-release metformin formulations are engineered using sophisticated pharmaceutical technologies designed to optimize glycemic control while minimizing gastrointestinal side effects. The most common system is the hydrophilic matrix tablet (as in Glucophage XR), which swells when exposed to gastrointestinal fluids, forming a gel layer that controls drug diffusion. Another technology is the osmotic pump system (as in Fortamet), which uses a semipermeable membrane surrounding the active drug core. Some products (like Glumetza) use gastric-retentive technology that expands in the stomach.
In osmotic pump tablets, water from the gastrointestinal tract enters through the semipermeable membrane, dissolving the drug core and creating pressure that forces the medication out through laser-drilled holes. The rate of release is controlled by the membrane's permeability and is largely independent of pH or gastrointestinal motility. After the medication has been fully released (release duration varies by product), the tablet shell—composed of cellulose acetate or similar materials—remains intact and is eliminated in the stool.
These delivery systems offer clinical advantages. Many extended-release formulations allow once-daily dosing compared to multiple daily doses for immediate-release metformin, potentially improving medication adherence. The gradual release also reduces peak plasma concentrations, which may decrease the incidence of dose-related gastrointestinal adverse effects such as diarrhea, nausea, and abdominal discomfort—common reasons for metformin discontinuation.
Patients should understand that seeing a tablet shell does not mean the medication was not absorbed. The shell is simply the delivery vehicle, and its presence in stool is an expected outcome of the controlled-release technology.
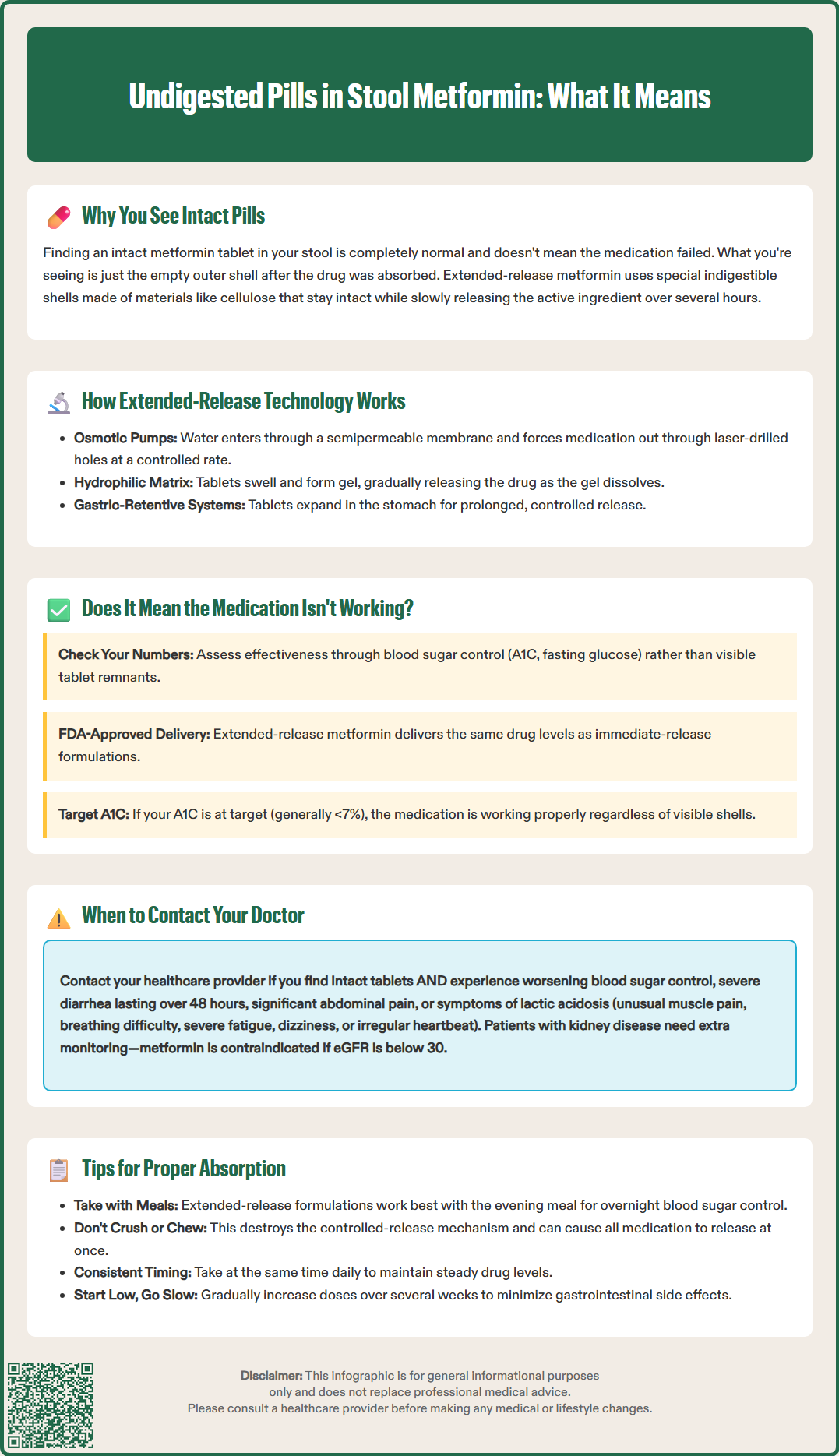
The appearance of tablet shells in stool does not automatically indicate treatment failure or inadequate drug absorption. Clinical efficacy should be assessed through objective measures rather than the presence of tablet remnants. The primary indicator of metformin effectiveness is glycemic control, measured by hemoglobin A1C, fasting plasma glucose, and postprandial glucose levels.
Pharmacological studies of extended-release metformin formulations have demonstrated comparable drug exposure (AUC) at equivalent total daily doses to immediate-release products for specific formulations, as noted in FDA labeling. The FDA approval process requires extensive pharmacokinetic data showing that extended-release formulations achieve therapeutic drug levels. If your blood glucose levels are well-controlled and your A1C is at target (generally <7% for many adults per American Diabetes Association guidelines), the medication is working regardless of visible tablet shells.
However, certain gastrointestinal conditions may genuinely impair metformin absorption. Patients with short bowel syndrome, severe inflammatory bowel disease with rapid transit, chronic diarrhea, or those who have undergone bariatric surgery may experience reduced drug absorption. In these cases, the medication may pass through the digestive system too quickly for adequate release and absorption, and immediate-release formulations or alternative diabetes medications may be more appropriate.
If you consistently see intact-appearing tablets and your blood glucose control has deteriorated—evidenced by rising fasting glucose readings, increased A1C levels, or symptoms of hyperglycemia such as increased thirst, frequent urination, or unexplained fatigue—this warrants medical evaluation. Your healthcare provider can assess whether the formulation is appropriate for your gastrointestinal function and may recommend switching to immediate-release metformin or adjusting your diabetes management regimen. Regular monitoring through laboratory testing remains the gold standard for evaluating medication effectiveness.
While finding tablet shells in stool is generally not concerning, certain situations require prompt medical attention. Contact your healthcare provider if you notice intact tablets accompanied by worsening glycemic control, as this combination may indicate inadequate drug absorption requiring formulation adjustment or alternative therapy.
You should seek medical advice if you experience new or worsening gastrointestinal symptoms alongside visible tablets, including:
Severe or persistent diarrhea lasting more than 48 hours, which may indicate medication intolerance or a gastrointestinal condition affecting absorption
Significant abdominal pain or cramping that interferes with daily activities
Nausea and vomiting preventing adequate food or fluid intake
Blood in stool or black, tarry stools, which may indicate gastrointestinal bleeding unrelated to metformin
Unexplained weight loss or signs of malabsorption
Immediate medical attention is warranted if you develop symptoms of lactic acidosis, a rare but serious metformin complication. Warning signs include unusual muscle pain, difficulty breathing, severe fatigue, dizziness, irregular heartbeat, or abdominal discomfort with nausea. Though lactic acidosis is uncommon in patients with normal kidney function, it represents a medical emergency requiring immediate evaluation.
Additionally, contact your provider if your home glucose monitoring reveals consistently elevated readings despite medication adherence, or if your most recent A1C test showed inadequate control. The American Diabetes Association recommends A1C testing every three months for patients not meeting glycemic targets.
Patients with kidney disease require particular vigilance. According to FDA guidance, metformin should not be initiated in patients with an estimated glomerular filtration rate (eGFR) below 45 mL/min/1.73m², and is contraindicated if eGFR is below 30 mL/min/1.73m². If you're scheduled for imaging with iodinated contrast, your provider may instruct you to temporarily stop metformin, especially if you have reduced kidney function (eGFR 30-60 mL/min/1.73m²), liver disease, alcoholism, or heart failure.
Optimizing metformin administration requires attention to timing, food intake, and proper tablet handling. These strategies can enhance medication effectiveness and minimize gastrointestinal side effects.
Take metformin with meals. Both immediate-release and extended-release formulations should be taken with food primarily to reduce gastrointestinal side effects. For extended-release formulations, taking the medication with the evening meal is often recommended per FDA labeling (e.g., Glucophage XR) to provide overnight glycemic control. While food slightly decreases the absorption of immediate-release metformin, this effect is not clinically significant, and the GI tolerability benefit outweighs this minor reduction.
Swallow tablets whole. Never crush, chew, or break extended-release metformin tablets, as this destroys the controlled-release mechanism and may cause all the medication to be released at once. This can lead to increased gastrointestinal side effects and potentially compromise glycemic control. If you have difficulty swallowing tablets, discuss alternatives with your healthcare provider, such as immediate-release tablets (which are smaller), liquid formulations, or different medication options.
Maintain consistent timing. Take metformin at the same time each day to maintain steady drug levels and establish a routine that supports adherence. For twice-daily dosing, space doses approximately 12 hours apart with morning and evening meals.
Stay well-hydrated. Adequate fluid intake supports proper gastrointestinal function and general health. Maintaining good hydration is important for all patients with diabetes.
Gradual dose titration helps minimize side effects. Healthcare providers typically start with low doses and increase gradually over several weeks, allowing your digestive system to adapt. If you experience persistent gastrointestinal symptoms, consult your provider before discontinuing medication—dose adjustment or formulation change may resolve the issue while maintaining glycemic control. Regular follow-up and open communication with your healthcare team ensure optimal diabetes management and medication effectiveness.
Yes, it is normal to see tablet shells from extended-release metformin in your stool. These are empty casings from the controlled-release delivery system, and their presence indicates the medication released properly rather than failed to work.
Switching formulations is unnecessary if your blood glucose and A1C levels remain well-controlled. The visible tablet shells are expected with extended-release formulations and do not indicate treatment failure unless accompanied by deteriorating glycemic control.
Contact your healthcare provider if you notice intact tablets alongside worsening blood glucose control or rising A1C levels. This combination may indicate inadequate absorption requiring formulation adjustment, particularly if you have gastrointestinal conditions affecting drug transit time.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.