LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Many patients who have undergone gallbladder removal wonder whether they can safely use weight loss injections for obesity management. The good news is that cholecystectomy does not prevent the use of GLP-1 receptor agonists like semaglutide (Wegovy) or tirzepatide (Zepbound). These FDA-approved medications work systemically through receptor activation rather than requiring gallbladder function, making them viable options for patients without this organ. However, individual considerations regarding digestive tolerance and monitoring requirements should be discussed with your healthcare provider to ensure safe and effective treatment tailored to your specific medical history.
Quick Answer: Yes, weight loss injections can be safely used after gallbladder removal, as there is no absolute contraindication to GLP-1 receptor agonist therapy in patients who have undergone cholecystectomy.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
Weight loss injections have emerged as effective pharmacological interventions for obesity management, with glucagon-like peptide-1 (GLP-1) receptor agonists representing the most widely prescribed class. These medications include semaglutide (Wegovy) and tirzepatide (Zepbound), which are FDA-approved for chronic weight management, while semaglutide (Ozempic) and tirzepatide (Mounjaro) are approved for type 2 diabetes management.
The mechanism of action involves multiple pathways. GLP-1 receptor agonists bind to receptors in the brain's appetite centers, particularly the hypothalamus, reducing hunger signals and increasing satiety. They also slow gastric emptying, which prolongs the feeling of fullness after meals. Additionally, these medications enhance insulin secretion in a glucose-dependent manner and suppress glucagon release, contributing to improved glycemic control in patients with type 2 diabetes.
Clinical trials have demonstrated substantial weight loss outcomes, with patients losing an average of approximately 15% of their body weight over 68 weeks with semaglutide 2.4 mg weekly in the STEP 1 trial. Tirzepatide, a dual GIP/GLP-1 receptor agonist, has shown even greater efficacy in some studies, with weight reductions of approximately 20% in the SURMOUNT-1 trial. These medications are administered via subcutaneous injection, once weekly for Wegovy and Zepbound.
The FDA has approved these weight management agents for adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity such as hypertension, type 2 diabetes, or dyslipidemia. They are intended as adjuncts to lifestyle modifications including reduced-calorie diet and increased physical activity, not as standalone treatments.
Important safety information includes a boxed warning for risk of thyroid C-cell tumors, making these medications contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. They are not recommended during pregnancy and should be discontinued if pregnancy occurs.
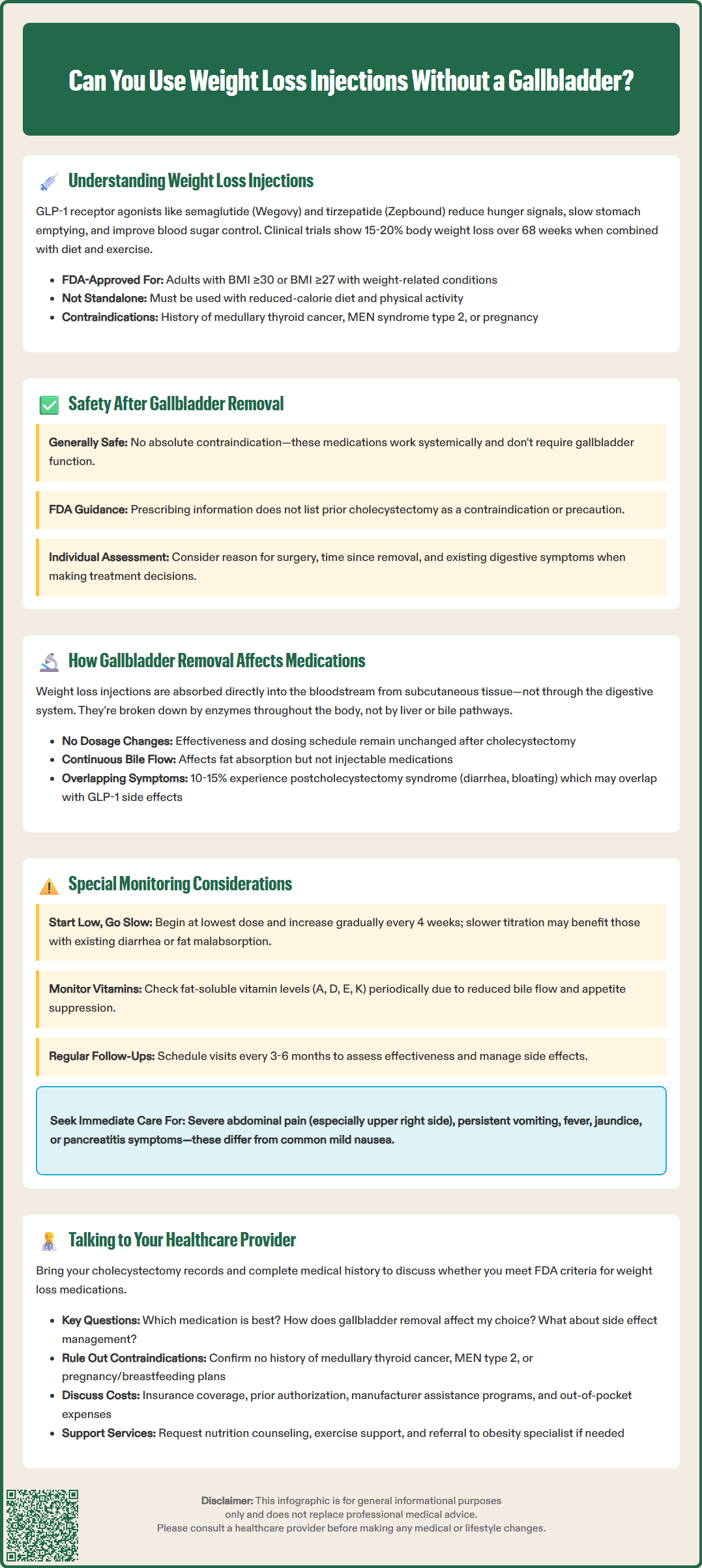
Patients who have undergone cholecystectomy (gallbladder removal) can generally use weight loss injections safely, as there is no absolute contraindication to GLP-1 receptor agonist therapy in this population. The gallbladder's primary function is bile storage and concentration, not bile production, which continues normally from the liver after cholecystectomy. Since weight loss medications work systemically through receptor activation rather than requiring gallbladder function, the absence of this organ does not fundamentally impair their therapeutic mechanism.
However, clinical considerations exist that warrant careful evaluation. Rapid weight loss from any cause, including pharmacotherapy, can increase the risk of gallstone formation in patients with intact gallbladders. For those who have already had cholecystectomy, the risk of gallbladder stones is eliminated, though bile duct stones (choledocholithiasis) can still occur, albeit less commonly. FDA labels for GLP-1 receptor agonists include warnings about increased risk of acute gallbladder disease, including cholecystitis and cholelithiasis, based on clinical trials and post-marketing data.
The FDA prescribing information for semaglutide and tirzepatide does not list prior cholecystectomy as a contraindication or precaution. Clinical trials of these medications have included participants with previous gallbladder removal, though specific safety outcomes in this subgroup have not been systematically reported. Individual patient factors—including the reason for cholecystectomy, time since surgery, and presence of ongoing digestive symptoms—should inform treatment decisions.
Patients without gallbladders may experience altered fat digestion and absorption, which could theoretically influence gastrointestinal tolerability of weight loss injections. The delayed gastric emptying caused by GLP-1 agonists might compound existing digestive changes, though this interaction has not been systematically studied in large populations. Patients should be monitored for both medication side effects and symptoms that could indicate biliary complications.
Cholecystectomy does not significantly alter the absorption or metabolism of weight loss injections because these medications are administered subcutaneously and bypass the gastrointestinal tract for initial absorption. GLP-1 receptor agonists are peptide-based biologics that are absorbed directly into the systemic circulation from subcutaneous tissue, making them independent of intestinal absorption processes that might be affected by altered bile flow.
The pharmacokinetics of injectable weight loss medications remain largely unchanged after gallbladder removal. These drugs are metabolized primarily through proteolytic degradation by peptidases throughout the body, not through hepatic cytochrome P450 enzymes or biliary excretion pathways. Semaglutide has an elimination half-life of approximately 168 hours, while tirzepatide's half-life is approximately 116-120 hours, both supporting once-weekly dosing. This pharmacokinetic profile is not influenced by gallbladder status.
What does change after cholecystectomy is the pattern of bile delivery to the intestine. Instead of concentrated bile released in response to meals, bile flows continuously from the liver into the duodenum. This can affect the digestion and absorption of dietary fats and fat-soluble vitamins (A, D, E, K), but does not impact the injectable medication itself. Some patients experience postcholecystectomy syndrome, characterized by diarrhea, bloating, or fat malabsorption, which occurs in approximately 10-15% of cases, though prevalence estimates vary.
The gastrointestinal side effects common with GLP-1 receptor agonists—including nausea, diarrhea, constipation, and abdominal discomfort—may overlap with or potentially exacerbate symptoms related to altered bile flow after cholecystectomy. However, there is no evidence that the medication's efficacy for weight loss or glycemic control is diminished in patients without gallbladders. Dose titration and monitoring protocols remain the same regardless of cholecystectomy history.
Patients without gallbladders who initiate weight loss injection therapy require individualized assessment and monitoring, though the fundamental approach mirrors that for patients with intact gallbladders. A thorough baseline evaluation should document the indication for cholecystectomy, time elapsed since surgery, and any persistent digestive symptoms. Patients who underwent cholecystectomy for acute cholecystitis or symptomatic cholelithiasis typically have uncomplicated courses, while those with biliary dyskinesia or postcholecystectomy syndrome may need closer attention.
Gastrointestinal tolerability deserves particular focus during dose titration. The standard approach involves starting at the lowest dose and gradually increasing every 4 weeks to minimize side effects. For patients with pre-existing diarrhea or fat malabsorption after cholecystectomy, slower titration schedules may improve adherence. Dietary modifications—including smaller, more frequent meals and reduced fat intake—can help manage both postcholecystectomy symptoms and medication-related gastrointestinal effects.
Monitoring should include assessment of nutritional status, particularly fat-soluble vitamin levels, as the combination of altered bile flow and reduced food intake from appetite suppression could theoretically increase deficiency risk. Baseline and periodic measurement of vitamins A, D, E, and K may be warranted in patients with documented malabsorption. Additionally, monitoring for signs of biliary complications remains prudent.
Patients should be counseled about distinguishing between expected medication side effects and symptoms requiring medical attention. Common GLP-1 agonist side effects like mild nausea typically improve over time. However, patients should seek urgent medical evaluation for severe or persistent abdominal pain (especially in the right upper quadrant), vomiting, fever, jaundice, or symptoms suggestive of pancreatitis. These may require laboratory testing (lipase, liver function tests) and imaging studies.
Regular follow-up visits every 3-6 months allow for efficacy assessment, side effect management, and adjustment of therapy as needed. Women of childbearing potential should use effective contraception during treatment, as these medications are not recommended during pregnancy and should be discontinued if pregnancy occurs.
Initiating a conversation about weight loss injections after cholecystectomy should begin with a comprehensive review of your medical history, including the circumstances surrounding gallbladder removal and your current health status. Bring documentation of your cholecystectomy, including operative reports if available, and be prepared to discuss any ongoing digestive symptoms, previous weight loss attempts, and current medications. Your healthcare provider will assess whether you meet FDA criteria for weight loss medication, which includes BMI thresholds and presence of weight-related comorbidities.
Key questions to discuss include the expected benefits and risks specific to your situation, alternative weight management strategies, and realistic expectations for weight loss outcomes. Ask about the specific medication being considered—whether semaglutide (Wegovy) or tirzepatide (Zepbound), which are FDA-approved for chronic weight management—and how your cholecystectomy history might influence the choice. Inquire about the titration schedule, injection technique training, and strategies for managing potential side effects. Understanding the long-term nature of treatment is essential, as these medications typically require ongoing use to maintain weight loss.
Your provider should evaluate contraindications and precautions, including personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, which are absolute contraindications to GLP-1 receptor agonists. History of pancreatitis, severe gastroparesis, or diabetic retinopathy (for patients with diabetes) requires careful consideration. If you are pregnant, planning pregnancy, or breastfeeding, these medications are not recommended. Insurance coverage and cost considerations are also important, as these medications can be expensive without adequate coverage. Discuss insurance prior authorization requirements and potential manufacturer assistance programs.
Establish a monitoring plan that includes regular follow-up visits, laboratory testing as indicated, and clear instructions for when to contact your healthcare team. Discuss integration with other aspects of your weight management program, including nutrition counseling, physical activity recommendations, and behavioral support. If your primary care provider is not comfortable prescribing these medications in the context of your cholecystectomy, request referral to an obesity medicine specialist or endocrinologist who can provide specialized expertise. Shared decision-making ensures that your treatment plan aligns with your goals, preferences, and individual medical circumstances.
No, gallbladder removal does not affect the efficacy of weight loss injections. These medications are administered subcutaneously and work through systemic receptor activation, independent of gallbladder function or bile storage.
There are no specific increased risks, though gastrointestinal side effects from the medication may overlap with postcholecystectomy digestive changes. Patients should be monitored for tolerability during dose titration, and bile duct stones remain a rare possibility even without a gallbladder.
The fundamental monitoring approach remains the same, though your provider may pay closer attention to gastrointestinal tolerability and nutritional status. Regular follow-up every 3-6 months, assessment of fat-soluble vitamin levels if malabsorption is present, and monitoring for biliary complications are recommended.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.