LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Does tirzepatide slow digestion? Yes, tirzepatide significantly delays gastric emptying as a core mechanism of action. This dual GIP and GLP-1 receptor agonist, marketed as Mounjaro for type 2 diabetes and Zepbound for chronic weight management, slows the movement of food from the stomach to the small intestine. This digestive effect contributes to prolonged satiety, reduced glucose spikes, and decreased caloric intake—but also accounts for the medication's most common side effects. Understanding how tirzepatide affects digestion helps patients and clinicians anticipate gastrointestinal changes and implement effective management strategies during treatment.
Quick Answer: Tirzepatide does slow digestion by delaying gastric emptying through its GLP-1 receptor agonist activity, causing food to move more slowly from the stomach into the small intestine.
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist with FDA approvals for two distinct indications: Mounjaro for type 2 diabetes management and Zepbound for chronic weight management in adults with BMI ≥30 kg/m² or ≥27 kg/m² with at least one weight-related comorbidity. This medication is not indicated for type 1 diabetes.
Yes, tirzepatide does slow digestion as part of its therapeutic mechanism. The GLP-1 component of tirzepatide delays gastric emptying, meaning food moves more slowly from the stomach into the small intestine. This delayed gastric emptying is a pharmacodynamic effect that contributes to several beneficial outcomes: prolonged satiety (feeling full longer), reduced postprandial glucose spikes, and decreased overall caloric intake. The medication also suppresses glucagon secretion when glucose levels are elevated and enhances insulin secretion in a glucose-dependent manner.
The slowing of gastric emptying is most pronounced during the initial weeks of treatment and typically attenuates somewhat over time as the body adapts. This effect contributes to both the therapeutic benefits and gastrointestinal side effects of tirzepatide. When used alone, tirzepatide has a low risk of hypoglycemia due to its glucose-dependent mechanism; however, this risk increases when combined with insulin or sulfonylureas, which may require dose adjustments of these medications.
The FDA-approved starting dose of 2.5 mg weekly is specifically designed to mitigate gastrointestinal effects during initiation and is not intended for long-term glycemic control. Understanding this mechanism helps patients and clinicians anticipate and manage the digestive changes that commonly occur during tirzepatide therapy.
Gastrointestinal adverse reactions are the most frequently reported effects of tirzepatide, directly related to its effect on gastric emptying and gut motility. The incidence varies by indication and dose, with higher rates generally observed in weight management trials compared to type 2 diabetes trials.
Common digestive side effects include:
Nausea (12-24% of patients): typically most prominent during dose escalation
Diarrhea (12-17% of patients): may alternate with constipation in some individuals
Decreased appetite: therapeutic effect but can be uncomfortable
Vomiting (6-10% of patients): usually transient and dose-related
Constipation (5-11% of patients): paradoxically occurs despite overall slowed transit
Abdominal pain or discomfort (6-10% of patients)
Dyspepsia and bloating: related to delayed gastric emptying
These effects are generally most pronounced during the first 4-8 weeks of treatment and during dose escalation periods. The FDA-approved dosing schedule includes gradual titration specifically to minimize gastrointestinal intolerance, starting at 2.5 mg weekly and increasing every 4 weeks as tolerated.
More serious but rare complications include acute pancreatitis (patients should discontinue tirzepatide if suspected and not restart if confirmed) and gallbladder disease. Severe gastrointestinal adverse reactions may lead to dehydration and acute kidney injury in some patients. Tirzepatide is not recommended for patients with severe gastrointestinal disease, including severe gastroparesis.
The slowed gastric emptying may affect the absorption of oral medications, particularly those requiring rapid absorption or those with narrow therapeutic windows. Notably, tirzepatide may decrease the effectiveness of oral contraceptives, especially during initiation and dose escalation periods. Women using oral contraceptives should consider using a non-oral method or adding a barrier method for 4 weeks after starting tirzepatide and after each dose increase.
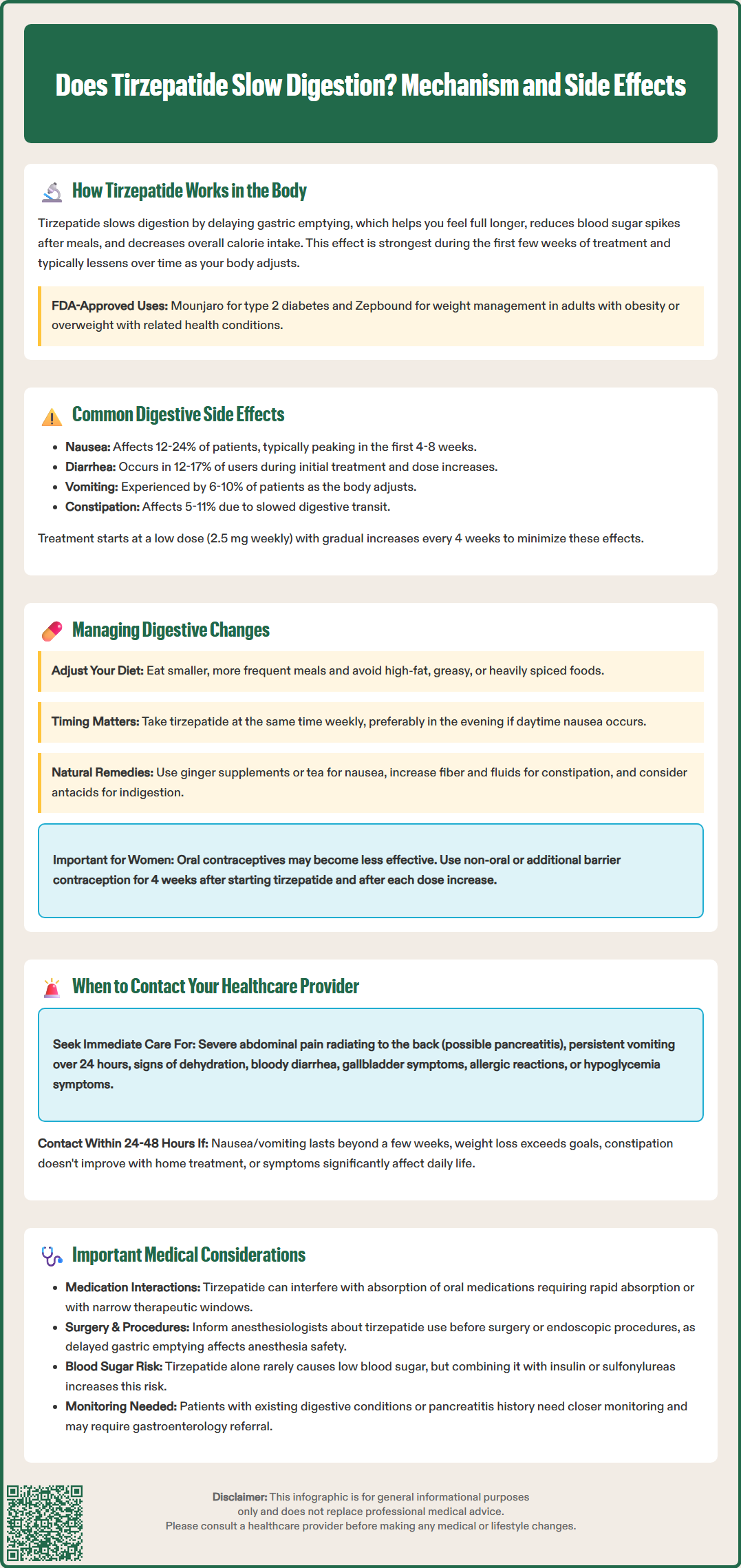
Effective management of digestive changes can significantly improve treatment adherence and patient comfort during tirzepatide therapy. Evidence-based strategies focus on dietary modifications, timing considerations, and symptomatic management.
Dietary modifications are the cornerstone of managing digestive side effects. Patients should consume smaller, more frequent meals rather than large portions, as the delayed gastric emptying means the stomach retains food longer. Avoiding high-fat, greasy, or heavily spiced foods can reduce nausea and discomfort, as these foods further slow gastric emptying. Adequate hydration is essential, particularly for patients experiencing diarrhea, though large volumes of liquid with meals may worsen bloating. Eating slowly and chewing thoroughly allows better tolerance of the slowed digestive process.
Timing and administration considerations include taking tirzepatide at a consistent time each week, preferably when patients can rest if nausea occurs. Some patients find that administering the injection in the evening reduces daytime nausea. The medication's dosing schedule is independent of meals.
Symptomatic management may include over-the-counter remedies for specific symptoms. Ginger supplements or ginger tea may help with nausea. For constipation, increased dietary fiber and adequate fluid intake are first-line approaches, with stool softeners or gentle laxatives if needed. Antacids may provide relief for dyspepsia. If conservative measures fail, healthcare providers may prescribe antiemetics such as ondansetron or consider temporarily holding the dose or slowing the titration schedule.
Patients should avoid lying down immediately after eating, as this can worsen reflux and nausea. Patients scheduled for surgery or endoscopic procedures should inform their anesthesiologist and proceduralist about tirzepatide use, as delayed gastric emptying may affect anesthesia safety and procedural planning.
Women using oral contraceptives should use a non-oral contraceptive method or add a barrier method for 4 weeks after starting tirzepatide and after each dose increase.
While most digestive side effects of tirzepatide are manageable and self-limiting, certain symptoms warrant prompt medical evaluation. Patients should be educated about warning signs that require clinical assessment to distinguish between expected side effects and potentially serious complications.
Seek immediate medical attention for:
Severe, persistent abdominal pain, particularly if radiating to the back, which may indicate pancreatitis
Persistent vomiting lasting more than 24 hours or inability to keep down fluids, risking dehydration
Signs of dehydration: decreased urination, dark urine, dizziness, dry mouth, or confusion
Severe diarrhea with blood, fever, or signs of volume depletion
Symptoms of gallbladder disease: right upper quadrant pain, jaundice, or clay-colored stools
Allergic reactions: rash, difficulty breathing, or swelling of the face or throat
Hypoglycemia symptoms (especially if taking insulin or sulfonylureas): shakiness, sweating, confusion, irritability, or dizziness
Contact your healthcare provider within 24-48 hours for:
Nausea or vomiting that persists beyond the first few weeks or significantly impacts nutrition
Unintentional weight loss exceeding clinical goals or signs of malnutrition
Persistent constipation unresponsive to conservative measures
New or worsening heartburn or reflux symptoms
Concerns about medication absorption due to vomiting or diarrhea
Symptoms that significantly impair quality of life or daily functioning
Patients with pre-existing gastrointestinal conditions or history of pancreatitis require closer monitoring. Persistent symptoms of early satiety, postprandial fullness, vomiting, or inability to advance diet may warrant gastroenterology referral and evaluation for gastroparesis (such as a gastric emptying study).
Healthcare providers may need to adjust the dose, slow the titration schedule, or consider alternative therapies based on individual tolerance. Regular follow-up appointments allow for assessment of treatment efficacy, side effect management, and appropriate dose optimization.
The slowing of gastric emptying is most pronounced during the initial weeks of treatment and typically attenuates somewhat over time as the body adapts, though the effect persists throughout therapy.
Yes, delayed gastric emptying may affect absorption of oral medications, particularly those requiring rapid absorption or with narrow therapeutic windows. Oral contraceptives may be less effective, requiring additional contraceptive methods for 4 weeks after starting tirzepatide and after each dose increase.
Consuming smaller, more frequent meals, avoiding high-fat and heavily spiced foods, eating slowly, staying well-hydrated, and not lying down immediately after eating can significantly reduce nausea, bloating, and discomfort.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.