LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Restarting tirzepatide after surgery requires careful clinical assessment and coordination between your surgical team and prescribing physician. While tirzepatide is often held before elective procedures due to its effects on gastric emptying, current guidance has evolved to support individualized decision-making based on patient-specific factors. The timing for safely resuming this dual GIP/GLP-1 receptor agonist depends on your recovery progress, return of normal gastrointestinal function, and ability to tolerate oral intake. Understanding when and how to restart tirzepatide ensures optimal diabetes management and weight control while minimizing post-operative complications.
Quick Answer: Tirzepatide can typically be restarted after surgery once normal gastrointestinal function returns, oral intake is well-tolerated, and the surgical team confirms adequate recovery.
We offer compounded medications and Zepbound®. Compounded medications are prepared by licensed pharmacies and are not FDA-approved. References to Wegovy®, Ozempic®, Rybelsus®, Mounjaro®, or Saxenda®, or other GLP-1 brands, are informational only. Compounded and FDA-approved medications are not interchangeable.
The timing for restarting tirzepatide after surgery depends on several clinical factors, including the type of procedure performed, the patient's recovery progress, and their ability to tolerate oral intake. Healthcare providers typically base the decision on clinical indicators rather than fixed timelines.
Key criteria for safely resuming tirzepatide include: return of normal gastrointestinal function (passing gas or having bowel movements), ability to tolerate regular diet without significant nausea or vomiting, stable hydration status, adequate pain control with minimal opioid requirements (as opioids can further slow gastric emptying), and stable vital signs.
Patients should consult their surgical team and prescribing physician before resuming tirzepatide, as individual circumstances vary considerably. The decision should be individualized based on surgical complexity, patient comorbidities, and post-operative recovery. For some patients, this may mean waiting only until oral intake is well-established, while others—particularly after complex procedures—may require a longer recovery period.
Patients should not restart tirzepatide and should contact their healthcare team if they experience: inability to keep fluids down for more than 4-6 hours, persistent vomiting for more than 24 hours, severe abdominal pain, or blood glucose levels consistently above 300 mg/dL.
Patients with diabetes may require alternative glucose management strategies during the interruption period. This might include more frequent blood glucose monitoring (fingerstick or continuous glucose monitoring) and temporary use of short-acting insulin or other antihyperglycemic agents according to their healthcare provider's recommendations.
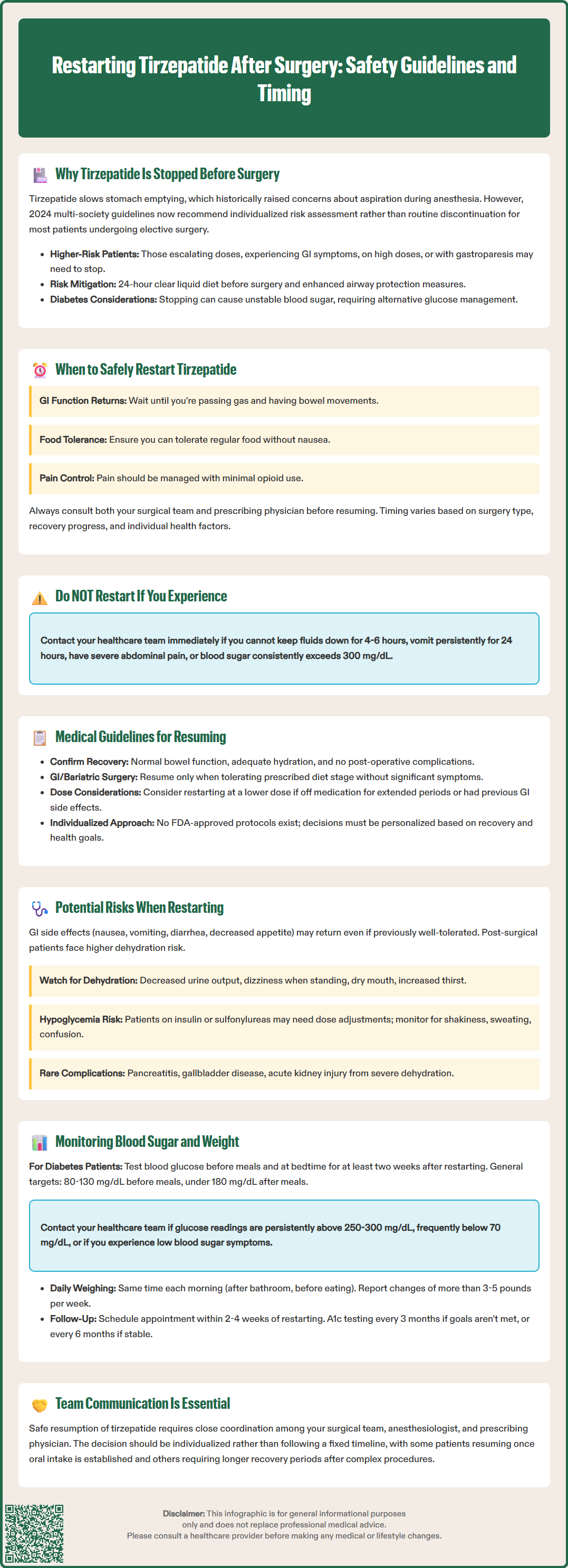
Tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, affects gastric emptying by slowing the movement of food through the stomach. This physiological effect has historically raised concerns about pulmonary aspiration—a serious complication where stomach contents enter the lungs during intubation or while under anesthesia.
Historically, the American Society of Anesthesiologists (ASA) had suggested holding GLP-1 receptor agonists prior to elective procedures requiring anesthesia. For weekly formulations like tirzepatide, discontinuation was typically advised at least one week before surgery, accounting for the medication's half-life of approximately 5 days.
However, current guidance has evolved. The 2024 multi-society statement (including the ASA) now advises that most patients can continue GLP-1 receptor agonists before elective surgery with appropriate risk stratification and mitigation strategies. Patients at higher risk—those in the dose-escalation phase, experiencing significant gastrointestinal symptoms, on high doses, or with gastroparesis—may still benefit from holding the medication. Risk mitigation strategies include a 24-hour clear liquid diet before surgery and enhanced airway protection measures.
Beyond aspiration risk, tirzepatide's glucose-lowering effects must be considered in the perioperative period. Patients with type 2 diabetes may experience fluctuating blood glucose levels when the medication is stopped, necessitating alternative management strategies.
Emergency surgeries present unique challenges, as there may be insufficient time for medication clearance, requiring modified anesthetic techniques and enhanced airway protection measures. The decision regarding tirzepatide management should be made collaboratively between the surgical team, anesthesiologist, and prescribing physician.
Current medical guidelines for resuming tirzepatide after surgery are evolving as clinical experience with GLP-1/GIP receptor agonists in the perioperative setting accumulates. The FDA-approved prescribing information for tirzepatide (Mounjaro, Zepbound) does not provide specific post-surgical restart protocols, leaving clinical decision-making to individual practitioners based on patient-specific factors.
Expert consensus suggests several principles for safely resuming tirzepatide: confirming return of normal bowel function, assessing the patient's ability to maintain hydration, and reviewing any post-operative complications that might contraindicate early resumption. These considerations align with general clinical practice for medications affecting gastrointestinal function.
For patients undergoing gastrointestinal surgery, particularly bariatric procedures or operations involving the stomach or upper intestinal tract, additional caution is warranted. These patients benefit from coordinated management between their surgeon and endocrinologist or prescribing physician. Tirzepatide should be resumed only as diet advances according to the surgical protocol and when the patient demonstrates tolerance of the prescribed diet stage without significant gastrointestinal symptoms.
If a patient has been off tirzepatide for an extended period or previously experienced significant gastrointestinal side effects, consider restarting at a lower dose than the pre-surgical maintenance dose, then titrating upward over subsequent weeks. This approach, consistent with FDA labeling recommendations for initial titration, may reduce the risk of gastrointestinal side effects in patients whose tolerance may have changed during the medication interruption.
Close communication between all members of the healthcare team ensures safe and effective resumption of therapy. The decision to restart should be individualized based on the patient's recovery status, diabetes control requirements, and weight management goals.
Restarting tirzepatide after a surgical interruption may be associated with a recurrence of gastrointestinal side effects, even in patients who previously tolerated the medication well. The most common adverse effects include nausea, vomiting, diarrhea, decreased appetite, and abdominal discomfort. These symptoms typically occur because the gastrointestinal system has had time to readjust during the medication-free period, and reintroduction can trigger a renewed adaptation response.
Patients recovering from surgery may be particularly vulnerable to dehydration if significant nausea or vomiting occurs upon restarting tirzepatide. Post-operative patients often have increased fluid requirements due to surgical stress, wound healing, and potential ongoing losses. Healthcare providers should counsel patients to monitor for signs of dehydration, including decreased urine output, dizziness upon standing, dry mucous membranes, and increased thirst.
Patients should seek urgent medical attention if they experience persistent vomiting for more than 24 hours, inability to keep fluids down for more than 4-6 hours, severe abdominal pain, signs of jaundice, or markedly decreased urine output. Severe or persistent gastrointestinal symptoms may necessitate temporary discontinuation or dose reduction.
Hypoglycemia represents another potential risk, primarily in patients taking tirzepatide in combination with insulin or sulfonylureas. When used as monotherapy, tirzepatide has a low risk of hypoglycemia. During the post-operative period, changes in dietary intake, physical activity levels, and metabolic stress can affect glucose homeostasis. When tirzepatide is reintroduced, dose adjustments to insulin or sulfonylureas may be necessary. Patients should be educated about hypoglycemia symptoms—shakiness, sweating, confusion, rapid heartbeat—and the importance of blood glucose monitoring.
Rare but serious adverse effects associated with tirzepatide include pancreatitis, gallbladder disease, and acute kidney injury secondary to severe dehydration. The post-operative period may mask or complicate the recognition of these conditions, making patient education and clinical vigilance essential.
Systematic monitoring of blood glucose levels and body weight is essential when restarting tirzepatide after surgery. Patients with type 2 diabetes should increase the frequency of self-monitoring blood glucose (SMBG) for at least the first two weeks after resuming treatment. Testing before meals and at bedtime helps identify patterns of hypoglycemia or hyperglycemia that may require medication adjustments.
Target glucose ranges should be individualized based on the patient's overall health status, diabetes duration, and presence of complications. The American Diabetes Association generally recommends preprandial glucose targets of 80–130 mg/dL and postprandial values less than 180 mg/dL for most adults with diabetes. During post-surgical recovery, slightly higher targets may be temporarily acceptable to minimize hypoglycemia risk, as determined by the healthcare provider. Patients should contact their healthcare team for glucose readings persistently above 250-300 mg/dL, frequent readings below 70 mg/dL, or any symptomatic hypoglycemia.
Continuous glucose monitoring (CGM) systems, when available, provide valuable real-time data and trend information that can guide therapy adjustments and identify problematic patterns that might be missed with intermittent fingerstick testing.
Weight monitoring serves multiple purposes in the post-operative period. Rapid weight loss may indicate inadequate nutritional intake or excessive fluid losses, while unexpected weight gain could suggest fluid retention or other complications. Patients should weigh themselves at the same time each day, preferably in the morning after voiding and before eating, and report significant changes (more than 3–5 pounds in a week) to their healthcare provider.
Healthcare providers should schedule follow-up appointments within 2 to 4 weeks of restarting tirzepatide to review glucose logs, assess weight trends, evaluate tolerance of the medication, and screen for adverse effects. Laboratory monitoring, including hemoglobin A1c, should be performed quarterly if therapy has changed or glycemic targets are not being met, and twice yearly if stable at goal. Other laboratory tests should be performed as clinically indicated rather than on a fixed schedule.
There is no fixed timeline for restarting tirzepatide after surgery. The decision depends on individual recovery factors including return of normal bowel function, ability to tolerate oral intake without significant nausea, stable hydration status, and clearance from your surgical team and prescribing physician.
Do not restart tirzepatide and contact your healthcare team if you cannot keep fluids down for more than 4-6 hours, experience persistent vomiting for over 24 hours, have severe abdominal pain, or have blood glucose levels consistently above 300 mg/dL.
Yes, gastrointestinal side effects such as nausea, vomiting, diarrhea, and decreased appetite may recur even if you previously tolerated tirzepatide well, as your system readjusts to the medication. Your healthcare provider may recommend restarting at a lower dose to minimize these effects.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.