LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Tirzepatide (Mounjaro, Zepbound) is a dual GIP and GLP-1 receptor agonist administered by subcutaneous injection for type 2 diabetes and chronic weight management. Some patients experience burning or stinging at the injection site, a recognized adverse reaction in FDA prescribing information. This discomfort typically results from the medication's formulation pH, tissue distension, or individual sensitivity. While injection site reactions occur in approximately 3.4% of patients in clinical trials, most cases are mild and resolve spontaneously. Understanding why tirzepatide burning at injection site occurs and implementing proper injection techniques can significantly improve patient comfort and treatment adherence.
Quick Answer: Tirzepatide burning at injection site is a recognized adverse reaction occurring in approximately 3.4% of patients, typically caused by the medication's formulation pH, tissue distension, or individual sensitivity, and usually resolves spontaneously within minutes to hours.
Several factors may potentially contribute to injection site burning. The medication's formulation is slightly alkaline compared to the skin's natural pH, which may cause transient irritation of subcutaneous tissues and nerve endings. Additionally, the volume injected and the administration via autoinjector may cause mechanical distension of tissue, potentially triggering discomfort. Medication injected directly from refrigerated storage might also intensify sensations by affecting local tissue response.
The subcutaneous injection itself involves depositing medication into adipose tissue, which contains sensory nerve fibers. Individual variation in pain perception, skin sensitivity, and local tissue characteristics can influence the intensity of burning sensations. Some patients may experience localized inflammatory responses, though true allergic reactions to tirzepatide are rare.
It is important to distinguish between expected injection site reactions and signs of infection or allergic response. Mild burning that resolves within minutes to hours is generally considered a normal reaction, whereas persistent pain, spreading redness, or systemic symptoms warrant clinical evaluation.
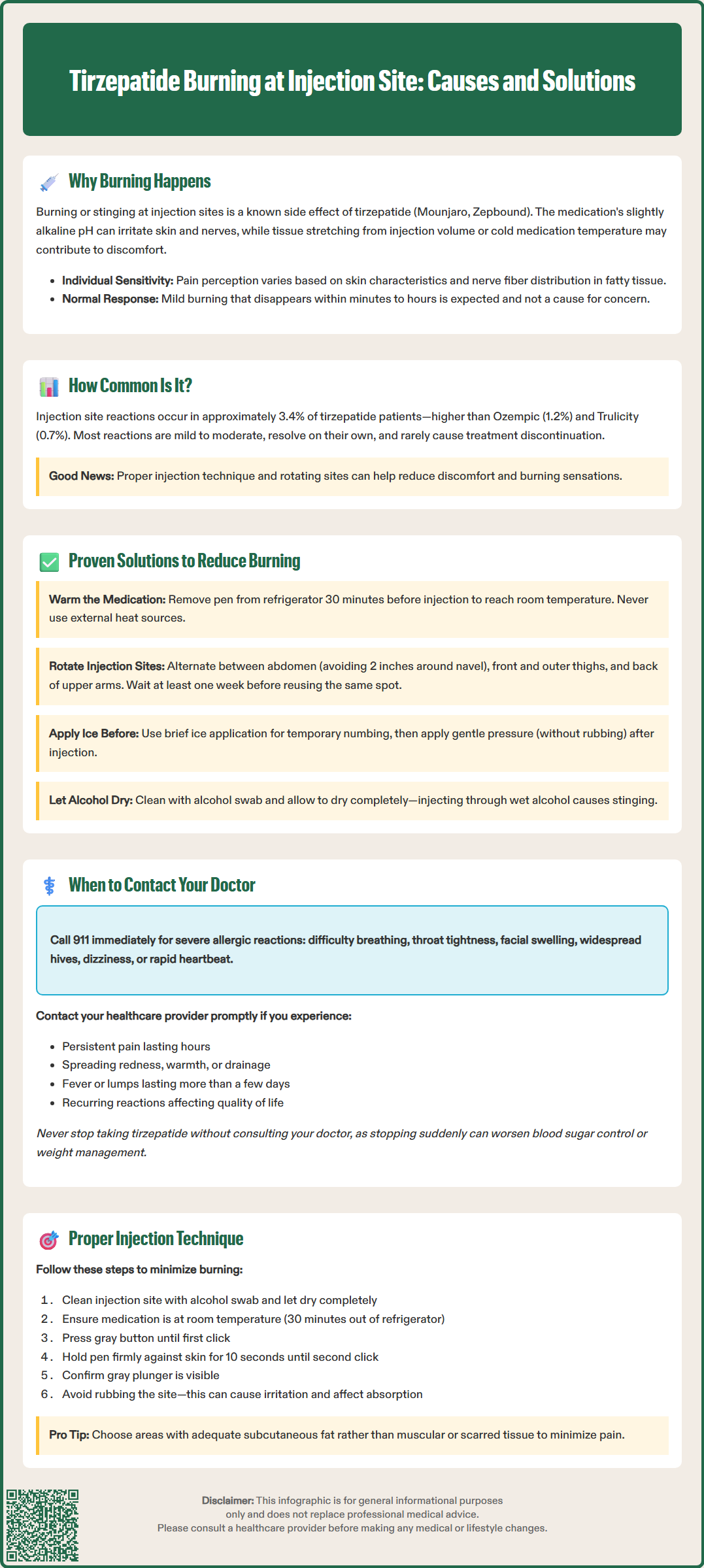
According to the FDA-approved prescribing information for tirzepatide, injection site reactions are among the reported adverse events. In the Mounjaro prescribing information, injection site reactions occurred in approximately 3.4% of patients receiving tirzepatide in clinical trials. The Zepbound prescribing information reports similar findings. However, specific data on burning sensations as a distinct symptom are not separately quantified in the prescribing information.
The SURPASS clinical trial program, which evaluated tirzepatide in patients with type 2 diabetes, documented injection site reactions as generally mild to moderate in severity. Most reactions resolved spontaneously without intervention and did not lead to treatment discontinuation. Some patients may experience more noticeable discomfort than others, reflecting individual variability in response to subcutaneous injections.
Other GLP-1 receptor agonists also report injection site reactions in their prescribing information. For example, the Ozempic (semaglutide) prescribing information notes injection site reactions in 1.2% of patients, while Trulicity (dulaglutide) reports injection site reactions in 0.7% of patients. Individual susceptibility varies considerably, with some patients experiencing minimal discomfort while others report more noticeable burning with injections.
Healthcare providers should counsel patients that mild, transient burning is a possible reaction and does not necessarily indicate a problem with the medication or injection technique. Many patients find that focusing on proper administration technique and site rotation helps manage these sensations.
Several strategies can help minimize burning and discomfort associated with tirzepatide injections. Implementing these techniques may improve patient tolerance and adherence to therapy.
Temperature management is an important consideration. According to the Instructions for Use, tirzepatide pens should be allowed to reach room temperature before injection. The Mounjaro and Zepbound Instructions for Use recommend taking the pen out of the refrigerator about 30 minutes before injecting. Cold medication may potentially increase discomfort. Patients should never use external heat sources or microwaves to warm the medication, as this may damage the medication.
Injection site rotation is essential for preventing localized tissue irritation. Recommended injection sites include the abdomen (avoiding a 2-inch radius around the navel), the front and outer thighs, and the back of the upper arms. Patients should avoid injecting into the same spot repeatedly and maintain a rotation schedule, waiting at least one week before returning to a previously used site. Injecting into areas with adequate subcutaneous fat, rather than muscular or scarred tissue, may also reduce discomfort.
Topical interventions may provide relief for some patients. Some find that applying ice to the injection site for a brief period before injection provides temporary numbing, though this should be balanced against the recommendation to use room-temperature medication. After injection, gentle pressure (without rubbing) can help reduce localized discomfort.
Following device instructions precisely is important. The tirzepatide autoinjector pen has specific administration steps outlined in the Instructions for Use, including proper placement against the skin and holding the device in place for the full 10 seconds after the second click is heard, ensuring complete medication delivery.
While mild, transient burning at the injection site is generally benign, certain signs and symptoms warrant prompt medical evaluation. Patients should be educated to recognize warning signs that may indicate infection, allergic reaction, or other complications requiring clinical assessment.
Call 911 or seek emergency medical attention immediately if you experience signs of a severe allergic reaction, including difficulty breathing, throat tightness, rapid swelling of the face or tongue, widespread hives, dizziness, or rapid heartbeat. Although rare, serious hypersensitivity reactions to tirzepatide have been reported and constitute a medical emergency.
Contact your healthcare provider promptly if you experience persistent or worsening pain at the injection site that does not resolve within a few hours, spreading redness or warmth extending beyond the immediate injection area, drainage or abscess formation, fever or systemic symptoms accompanying injection site reactions, or hardened nodules or lumps that persist for more than a few days. These findings may indicate an infection or other complication requiring medical evaluation.
Routine follow-up is appropriate for recurrent injection site reactions that, while not severe, significantly impact quality of life or treatment adherence. Healthcare providers can assess injection technique, review site rotation practices, and consider whether alternative treatment options might be better tolerated. Persistent reactions may also prompt evaluation for underlying conditions affecting wound healing or immune function.
Patients should never discontinue tirzepatide without consulting their healthcare provider, as abrupt cessation may lead to deterioration in glycemic control or weight management. If injection site reactions are problematic, providers can work with patients to optimize technique or explore alternative therapeutic options while maintaining treatment continuity.
Mastering proper injection technique is fundamental to minimizing discomfort and ensuring optimal medication delivery. Healthcare providers should provide training at treatment initiation, with periodic reassessment to reinforce correct practices.
Pre-injection preparation begins with hand hygiene using soap and water or alcohol-based sanitizer. The injection site should be cleaned with an alcohol swab and allowed to dry completely—injecting through wet alcohol can cause significant stinging. Patients should inspect the medication for particulates or discoloration and verify the expiration date. The pen should be at room temperature, having been removed from refrigeration approximately 30 minutes prior, per the Instructions for Use.
Injection technique for tirzepatide autoinjector pens (Mounjaro/Zepbound) follows specific steps outlined in the manufacturer's Instructions for Use:
Remove the pen cap and verify the medication appears clear and colorless to slightly yellow
Select and clean an appropriate injection site (abdomen, thigh, or back of upper arm)
Place the clear base flat against the skin at the injection site
Unlock the pen by turning the lock ring
Press and hold the gray injection button; you will hear a click
Continue holding the button and the pen firmly against your skin for 10 seconds until you hear a second click
Remove the pen from your skin and confirm the gray plunger is visible in the window, indicating the dose was delivered
Post-injection care: Apply gentle pressure with a clean gauze pad if needed, but avoid rubbing the site, which can cause irritation and affect medication absorption. Properly dispose of the used pen in a puncture-resistant container.
Common technique errors that may increase discomfort include using cold medication, inadequate site rotation, injecting into areas with insufficient subcutaneous fat, and injecting through wet alcohol. Healthcare providers should periodically review injection technique with patients to identify and address any issues. Many patients benefit from reviewing the Instructions for Use or instructional videos provided by the manufacturer.
Yes, mild burning or stinging at the injection site is a recognized adverse reaction that occurs in approximately 3.4% of patients. Most cases are mild, resolve within minutes to hours, and do not require treatment discontinuation.
Allow the pen to reach room temperature for 30 minutes before injection, rotate injection sites regularly, ensure the alcohol swab dries completely before injecting, and follow the manufacturer's Instructions for Use precisely. These techniques can significantly reduce injection site discomfort.
Contact your healthcare provider for persistent pain lasting more than a few hours, spreading redness or warmth, drainage, fever, or hardened lumps. Seek emergency care immediately for signs of severe allergic reaction such as difficulty breathing, throat tightness, or rapid facial swelling.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.