LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Can semaglutide cause thyroid issues? This question concerns many patients considering this GLP-1 receptor agonist for type 2 diabetes or weight management. Semaglutide (Ozempic, Wegovy, Rybelsus) carries an FDA boxed warning about thyroid C-cell tumors observed in rodent studies, including medullary thyroid carcinoma. However, no conclusive evidence exists that semaglutide increases thyroid cancer risk in humans. The medication is contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. Understanding these precautions helps patients and clinicians make informed treatment decisions while recognizing that millions have used semaglutide safely without established thyroid complications.
Quick Answer: Semaglutide caused thyroid C-cell tumors in rodent studies, but no conclusive evidence exists that it increases medullary thyroid carcinoma risk in humans.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for type 2 diabetes management (under the brand names Ozempic and Rybelsus) and chronic weight management (as Wegovy). While this medication has demonstrated significant benefits for glycemic control and weight reduction, it carries a boxed warning—the FDA's most serious safety alert—regarding thyroid C-cell tumors observed in animal studies.
The thyroid safety concern stems from preclinical research in rodents, where semaglutide caused dose-dependent and treatment-duration-dependent thyroid C-cell tumors, including medullary thyroid carcinoma (MTC). These C-cells produce calcitonin, a hormone involved in calcium regulation. In rats and mice exposed to semaglutide at clinically relevant doses, researchers observed C-cell adenomas and carcinomas at statistically significant rates compared to control animals.
It is important to understand that no conclusive evidence exists that semaglutide increases MTC risk in humans; the human relevance remains unknown. GLP-1 receptors are expressed differently across species. Human thyroid C-cells have substantially lower GLP-1 receptor density compared to rodents, which may reduce susceptibility to GLP-1-mediated proliferative effects. Despite extensive clinical use and post-marketing surveillance involving millions of patients worldwide, a causal link between semaglutide and thyroid C-cell tumors in humans has not been established.
Nonetheless, the FDA requires that prescribing information include this boxed warning as a precautionary measure. Healthcare providers should counsel patients about this theoretical risk before initiating treatment, particularly those with personal or family histories of thyroid malignancy or multiple endocrine neoplasia syndromes. Patients also receive this information in the required Medication Guide.
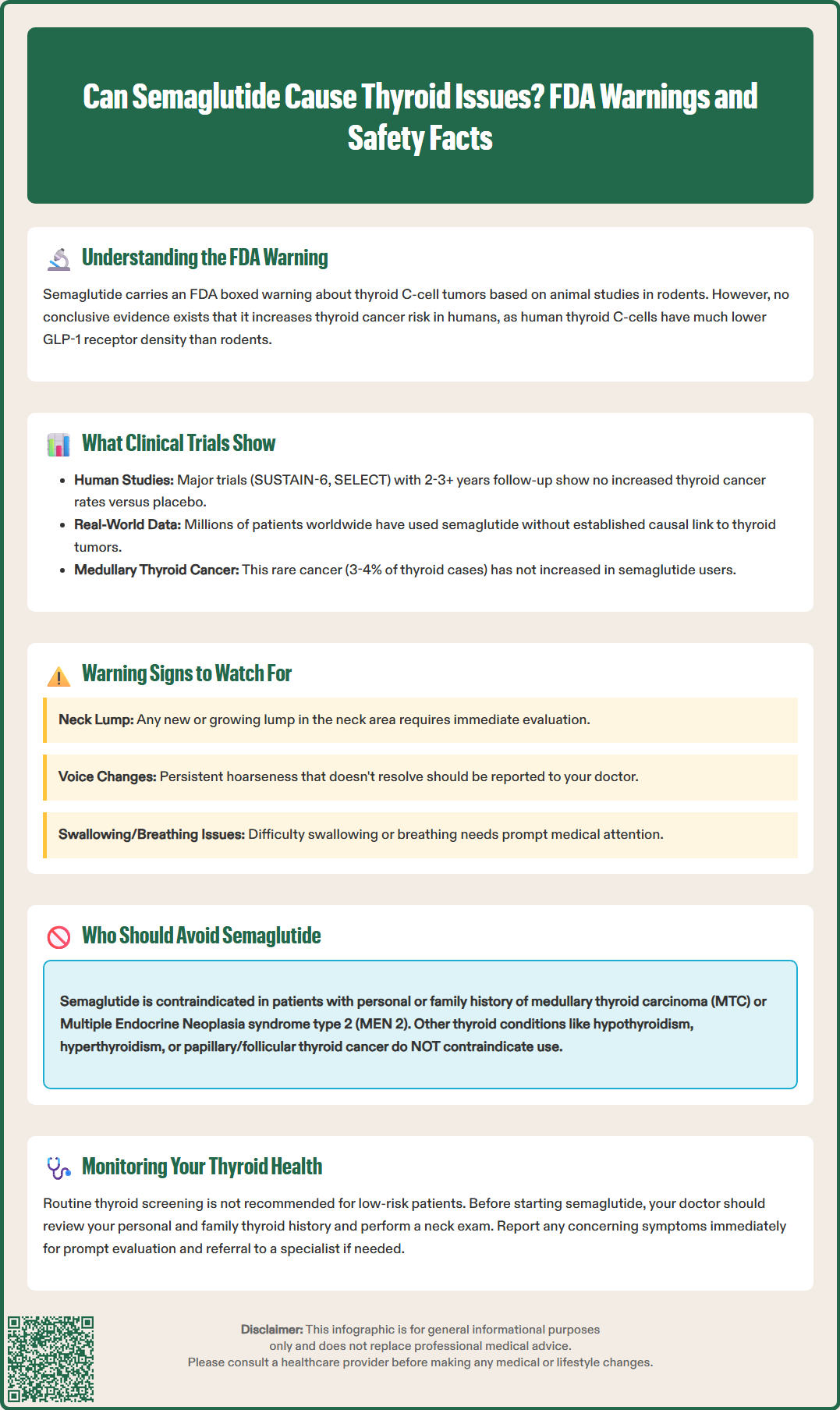
The FDA's boxed warning for semaglutide explicitly states that the drug has been shown to cause thyroid C-cell tumors in rodent studies and is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). This warning applies to several long-acting GLP-1 receptor agonists, including semaglutide (Ozempic, Wegovy, Rybelsus), liraglutide (Victoza, Saxenda), dulaglutide (Trulicity), and exenatide extended-release (BYDUREON BCise). Short-acting agents like exenatide immediate-release (Byetta) and lixisenatide (Adlyxin) do not carry this warning. Tirzepatide (Mounjaro, Zepbound), a dual GIP/GLP-1 receptor agonist, also carries a similar boxed warning.
Medullary thyroid carcinoma is a rare form of thyroid cancer, accounting for approximately 3-4% of all thyroid malignancies in the United States. It originates from parafollicular C-cells and often presents with elevated serum calcitonin levels. MEN 2 is a hereditary syndrome characterized by MTC, pheochromocytoma, and parathyroid abnormalities, caused by mutations in the RET proto-oncogene. Patients with MEN 2 have substantially elevated lifetime risk of developing MTC, often at younger ages.
The FDA's decision to include this boxed warning reflects the agency's conservative approach to potential carcinogenic signals, even when human relevance is uncertain. Regulatory guidance requires that serious findings in animal studies be communicated to prescribers and patients, allowing for informed decision-making. The warning emphasizes that semaglutide is contraindicated in individuals with these specific risk factors, meaning it should not be prescribed under any circumstances to this population.
Clinical trials of semaglutide, including the SUSTAIN and STEP programs, have not demonstrated increased incidence of thyroid cancer compared to placebo or active comparators. The SUSTAIN-6 cardiovascular outcome trial, with approximately 2.1 years of follow-up, and the SELECT trial, with over 3 years of follow-up, have not identified safety signals for thyroid malignancy. Post-marketing pharmacovigilance data similarly have not established a causal link between semaglutide use and MTC in humans, though ongoing monitoring continues.
Patients taking semaglutide should be educated about symptoms that may indicate thyroid pathology, particularly those suggestive of medullary thyroid carcinoma, though such occurrences would be extremely rare. Key warning signs include a palpable neck mass or lump, persistent hoarseness or voice changes, difficulty swallowing (dysphagia), or difficulty breathing (dyspnea). These symptoms may indicate a thyroid nodule or mass effect from an enlarging thyroid lesion.
Medullary thyroid carcinoma often presents as a painless thyroid nodule, which may be detected by the patient or during physical examination. Unlike more common thyroid conditions, MTC does not typically cause the classic symptoms of thyroid hormone imbalance. However, patients should also be aware of general thyroid dysfunction symptoms. Hypothyroidism symptoms include unexplained fatigue, cold intolerance, constipation, dry skin, weight gain despite reduced appetite, and cognitive slowing. Hyperthyroidism symptoms include anxiety, tremor, heat intolerance, palpitations, unintentional weight loss, and increased bowel frequency.
It is important to note that semaglutide itself does not directly affect thyroid hormone production or cause primary thyroid dysfunction such as hypothyroidism or hyperthyroidism. The medication's mechanism of action—enhancing glucose-dependent insulin secretion, suppressing glucagon, and slowing gastric emptying—does not involve the hypothalamic-pituitary-thyroid axis. However, oral semaglutide (Rybelsus) can increase levothyroxine exposure, so TSH should be monitored when these medications are co-administered, and timing or dosage adjustments may be needed.
Patients experiencing any concerning neck symptoms should promptly contact their healthcare provider for evaluation. While the absolute risk of thyroid malignancy remains very low, early detection of any thyroid pathology improves outcomes. Concerning symptoms or the discovery of a thyroid nodule warrant ultrasound evaluation and potential referral to an endocrinologist or thyroid specialist.
Semaglutide carries absolute contraindications related to thyroid cancer risk that must be strictly observed. Patients with a personal history of medullary thyroid carcinoma (MTC) should never receive semaglutide, regardless of treatment status or time since diagnosis. This contraindication reflects the theoretical concern that GLP-1 receptor stimulation could promote recurrence or progression of residual C-cell disease, even though human C-cells express minimal GLP-1 receptors.
Patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) are also absolutely contraindicated from receiving semaglutide. MEN 2 encompasses two subtypes: MEN 2A (characterized by MTC, pheochromocytoma, and primary hyperparathyroidism) and MEN 2B (characterized by MTC, pheochromocytoma, marfanoid habitus, and mucosal neuromas). Individuals with MEN 2 carry germline RET mutations conferring near-100% lifetime risk of developing MTC, often presenting in childhood or early adulthood. Exposing these patients to a medication with even theoretical C-cell proliferative potential is considered unacceptable risk.
Any family history of MTC also represents a contraindication to semaglutide use, not limited to first-degree relatives. Approximately 25% of MTC cases are hereditary, associated with MEN 2 syndromes or familial MTC without other endocrine manifestations. Before prescribing semaglutide, clinicians should specifically inquire about family history of thyroid cancer, distinguishing MTC from the more common papillary or follicular thyroid cancers, which do not contraindicate GLP-1 receptor agonist therapy.
Patients with a history of other thyroid conditions—such as papillary thyroid cancer, follicular thyroid cancer, thyroid nodules, hypothyroidism, or hyperthyroidism—are not contraindicated from receiving semaglutide based on thyroid history alone. These conditions do not involve C-cells and do not increase susceptibility to potential GLP-1-mediated effects. However, comprehensive medical history and individualized risk-benefit assessment remain essential components of prescribing decisions.
Current FDA-approved prescribing information for semaglutide does not require routine thyroid monitoring, including serum calcitonin measurements or thyroid ultrasound surveillance, in patients without risk factors. This recommendation reflects the lack of established human risk and the limitations of screening tests. Routine calcitonin screening in the general population has poor positive predictive value, as calcitonin elevations occur in numerous benign conditions including C-cell hyperplasia, chronic kidney disease, proton pump inhibitor use, and thyroiditis. False-positive results could lead to unnecessary anxiety and invasive procedures.
However, clinicians should conduct a thorough baseline assessment before initiating semaglutide. This includes detailed personal and family history specifically addressing thyroid malignancy, particularly MTC and MEN 2 syndromes. A careful physical examination should include neck palpation to identify pre-existing thyroid nodules or masses. If clinical suspicion exists based on history or examination findings, appropriate evaluation—potentially including thyroid ultrasound, serum calcitonin, or genetic testing for RET mutations—should be completed before starting therapy.
During ongoing treatment, patients should be counseled to promptly report any symptoms such as neck masses, hoarseness, or difficulty swallowing or breathing, as outlined in the Medication Guide. Healthcare providers should evaluate these symptoms if they arise. If a suspicious thyroid nodule is found or if calcitonin is substantially elevated (e.g., >50-100 pg/mL, depending on laboratory reference ranges), referral to an endocrinologist or thyroid specialist is warranted for ultrasound evaluation, possible fine-needle aspiration, and consideration of RET mutation testing if familial risk is suspected.
Patient education represents a critical prevention strategy. Before prescribing semaglutide, clinicians should discuss the boxed warning in understandable terms, explaining that animal studies showed thyroid tumors but human risk remains theoretical and unproven. Patients should understand the absolute contraindications and the importance of disclosing complete family history. Shared decision-making should weigh the established benefits of semaglutide for diabetes control and weight management against theoretical thyroid risks, particularly in patients with multiple cardiovascular risk factors who stand to benefit substantially from treatment. For patients with significant anxiety about thyroid risk despite reassurance about the lack of human evidence, alternative therapeutic options should be considered.
No conclusive evidence exists that semaglutide causes thyroid cancer in humans. While rodent studies showed thyroid C-cell tumors, clinical trials and post-marketing surveillance involving millions of patients have not established a causal link between semaglutide and medullary thyroid carcinoma in humans.
Semaglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. Patients with other thyroid conditions like papillary thyroid cancer, hypothyroidism, or thyroid nodules are not contraindicated based on thyroid history alone.
Report any neck mass or lump, persistent hoarseness or voice changes, difficulty swallowing, or difficulty breathing to your healthcare provider immediately. These symptoms may indicate thyroid pathology requiring evaluation, though such occurrences are extremely rare.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.