LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Tirzepatide (Mounjaro for type 2 diabetes, Zepbound for weight management) is a dual GIP/GLP-1 receptor agonist that has transformed diabetes and obesity treatment. As use expands, patients and clinicians are exploring potential connections between tirzepatide and panic attacks or anxiety symptoms. While current FDA labeling and clinical trial data do not establish a direct causal link between tirzepatide and panic disorder, understanding the medication's effects, potential indirect mechanisms, and appropriate management strategies is essential for safe, effective treatment. This article examines the evidence, explores possible contributing factors, and provides guidance for patients experiencing anxiety-related symptoms during tirzepatide therapy.
Quick Answer: Current FDA-approved labeling and clinical trial data do not establish a causal relationship between tirzepatide and panic attacks.
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved by the FDA for the treatment of type 2 diabetes mellitus (brand name Mounjaro) and chronic weight management (brand name Zepbound). As a dual incretin mimetic, tirzepatide works through multiple complementary mechanisms to improve glycemic control and promote weight loss.
The medication enhances glucose-dependent insulin secretion from pancreatic beta cells, meaning it stimulates insulin release only when blood glucose levels are elevated, thereby reducing the risk of hypoglycemia. Simultaneously, tirzepatide suppresses inappropriate glucagon secretion, slows gastric emptying to prolong satiety, and acts on central appetite regulation pathways in the hypothalamus to reduce food intake. These combined effects result in improved hemoglobin A1c levels, significant weight reduction, and favorable changes in cardiovascular risk factors including blood pressure and lipid profiles, though cardiovascular outcomes benefits have not yet been established.
Tirzepatide is administered as a once-weekly subcutaneous injection, with dosing typically initiated at 2.5 mg and gradually titrated upward (5 mg, 7.5 mg, 10 mg, 12.5 mg, to a maximum of 15 mg) based on glycemic response and tolerability. The gradual dose escalation helps minimize gastrointestinal side effects, which represent the most commonly reported adverse reactions. Clinical trials have demonstrated substantial efficacy, with patients achieving A1c reductions of 1.9% to 2.4% in type 2 diabetes and weight loss averaging 15% to 21% of baseline body weight after 72 weeks in adults without diabetes (more modest in those with type 2 diabetes). Important safety considerations include a contraindication in patients with personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN2). Understanding tirzepatide's mechanism of action provides important context when evaluating its potential effects on mental health and anxiety-related symptoms.
Currently, there is no established causal link between tirzepatide and panic attacks in the FDA-approved prescribing information or published clinical trial data. The pivotal SURPASS clinical trial program, which enrolled thousands of patients with type 2 diabetes, did not identify anxiety disorders or panic attacks as significant adverse events attributable to tirzepatide therapy. Similarly, the SURMOUNT trials evaluating tirzepatide for weight management did not report increased rates of panic symptoms compared to placebo, though it's important to note that panic symptoms were not prespecified outcomes in these studies.
However, several indirect mechanisms warrant clinical consideration. Gastrointestinal adverse effects—including nausea (occurring in up to 43% of patients at higher doses), vomiting, diarrhea, and abdominal discomfort—may trigger or exacerbate anxiety symptoms in susceptible individuals. The physical sensations associated with nausea and gastric distress can mimic or precipitate panic-like symptoms, particularly in patients with pre-existing anxiety disorders. Additionally, rapid weight loss and significant dietary changes may affect nutritional status, energy levels, and psychological well-being, potentially influencing mood and anxiety regulation.
Hypoglycemia, though uncommon with tirzepatide monotherapy, can occur when combined with insulin or sulfonylureas. Hypoglycemic episodes produce physiological symptoms—tremor, palpitations, sweating, and confusion—that closely resemble panic attacks and may be misinterpreted as such. Furthermore, patients with diabetes and obesity experience higher baseline rates of depression and anxiety compared to the general population, independent of medication use.
It's important to note that the FDA-approved labeling for Zepbound (tirzepatide for weight management) includes a warning to monitor for depression, suicidal thoughts, or suicidal behavior. While the FDA has stated that current data do not demonstrate a causal link between GLP-1 receptor agonists and suicidal thoughts, healthcare providers should remain vigilant. Clinicians should maintain awareness of these possibilities while recognizing that current evidence does not support a direct pharmacological mechanism linking tirzepatide to panic disorder.
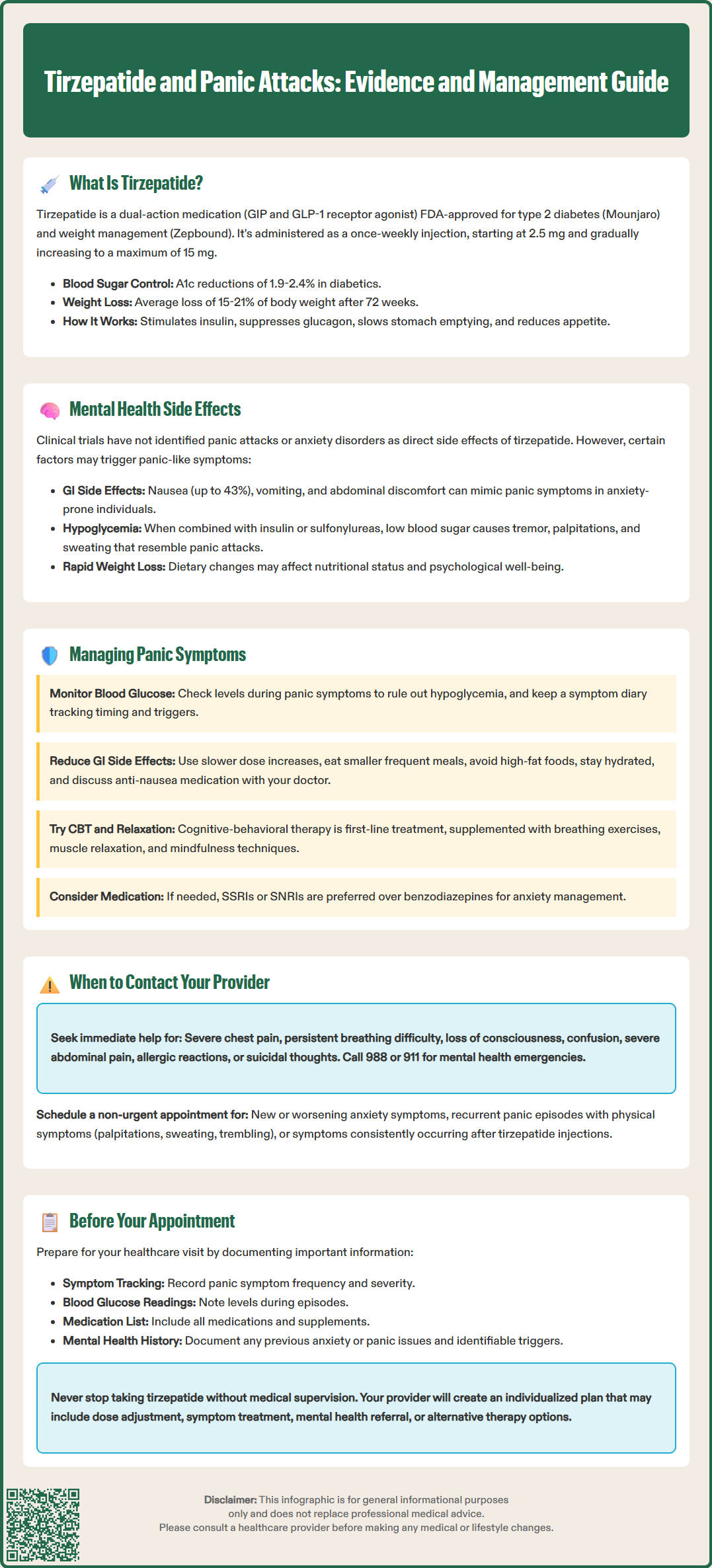
If panic symptoms emerge during tirzepatide therapy, a systematic approach to evaluation and management is essential. First, clinicians should conduct a thorough assessment to differentiate true panic attacks from physiological mimics. Checking blood glucose levels during symptomatic episodes helps exclude hypoglycemia, particularly in patients receiving concomitant insulin or sulfonylureas. A detailed symptom diary documenting the timing, duration, triggers, and associated physical sensations can clarify whether symptoms correlate with medication administration, meals, or gastrointestinal side effects.
Optimizing gastrointestinal tolerability may alleviate anxiety-like symptoms if nausea or abdominal discomfort contributes to distress. Strategies include slower dose titration, eating smaller and more frequent meals (especially on injection days), avoiding high-fat foods that delay gastric emptying further, and ensuring adequate hydration. If nausea persists, antiemetic medications such as ondansetron may provide symptomatic relief, though this should be discussed with the prescribing physician.
For patients with confirmed panic symptoms, evidence-based psychological interventions represent first-line management. Cognitive-behavioral therapy (CBT) specifically designed for panic disorder demonstrates robust efficacy and should be considered regardless of medication continuation. Breathing exercises, progressive muscle relaxation, and mindfulness techniques can help manage acute panic episodes. If pharmacological treatment for anxiety becomes necessary, selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are preferred, with consideration for more weight-neutral options when possible. Benzodiazepines carry risks of dependence and may complicate diabetes management through effects on cognition and fall risk.
Patients should maintain open communication with their healthcare team, reporting any new or worsening mental health symptoms promptly. In some cases, temporarily reducing the tirzepatide dose or extending the interval between dose increases may help determine whether symptoms are medication-related. For suicidal thoughts or behaviors, call the 988 Suicide & Crisis Lifeline immediately or 911 if in imminent danger. Never discontinue tirzepatide without medical guidance, as abrupt cessation may lead to deterioration in glycemic control or weight regain.
Patients experiencing panic-like symptoms while taking tirzepatide should contact their healthcare provider if symptoms are severe, persistent, or significantly impair daily functioning. Immediate medical attention is warranted for symptoms suggesting serious complications, including severe chest pain, difficulty breathing that does not resolve with calming techniques, loss of consciousness, or confusion that may indicate severe hypoglycemia or other acute medical conditions requiring urgent evaluation.
Seek emergency care immediately for severe, persistent abdominal pain (with or without vomiting) which may indicate pancreatitis; right upper quadrant pain, fever, or yellowing of the skin/eyes which could suggest gallbladder disease; or signs of allergic reaction such as rash, itching, swelling, or difficulty breathing. If experiencing suicidal thoughts or behaviors, call the 988 Suicide & Crisis Lifeline or 911 if in immediate danger.
Schedule a non-urgent appointment if you experience new or worsening anxiety symptoms, recurrent episodes of intense fear or discomfort accompanied by physical symptoms (palpitations, sweating, trembling, shortness of breath), persistent nausea or vomiting that interferes with medication adherence or nutrition, or symptoms that consistently occur in temporal relationship to tirzepatide injections. Additionally, contact your provider if you have a history of panic disorder or anxiety that appears to be worsening since starting tirzepatide, or if you are considering discontinuing the medication due to mental health concerns.
Before your appointment, prepare relevant information to facilitate clinical assessment. Document the frequency, duration, and severity of panic symptoms, noting any identifiable triggers or patterns. Record blood glucose readings, particularly during symptomatic episodes, and maintain a list of all medications, supplements, and recent dosage changes. Be prepared to discuss your mental health history, including any previous anxiety or panic disorder diagnoses, prior treatments, and current stressors or life changes that may contribute to symptoms.
Your healthcare provider will conduct a comprehensive evaluation to determine whether symptoms are related to tirzepatide, represent an underlying or co-existing condition, or result from other factors. This assessment may include physical examination, laboratory testing (including thyroid function and glucose monitoring), review of medication interactions, and mental health screening. Based on these findings, your provider will develop an individualized management plan that may include dose adjustment, symptomatic treatment, mental health referral, or consideration of alternative diabetes or weight management therapies. Never discontinue tirzepatide without medical supervision, as this decision requires careful consideration of the benefits of continued therapy against the impact of reported symptoms.
No, current FDA labeling and clinical trial data from the SURPASS and SURMOUNT programs do not establish a direct causal link between tirzepatide and panic attacks. However, indirect mechanisms such as gastrointestinal side effects or hypoglycemia may trigger anxiety-like symptoms in susceptible individuals.
Contact your healthcare provider for a comprehensive evaluation to differentiate true panic attacks from physiological mimics like hypoglycemia. Document symptom timing, check blood glucose during episodes, and discuss whether dose adjustment, symptomatic treatment, or mental health referral is appropriate. Never discontinue tirzepatide without medical supervision.
Yes, gastrointestinal adverse effects such as nausea, vomiting, and abdominal discomfort can trigger or exacerbate anxiety symptoms in susceptible individuals. The physical sensations associated with gastric distress may mimic or precipitate panic-like symptoms, particularly in patients with pre-existing anxiety disorders.
All medical content on this blog is created using reputable, evidence-based sources and is regularly reviewed for accuracy and relevance. While we strive to keep our content current with the latest research and clinical guidelines, it is intended for general informational purposes only.
This content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a licensed healthcare provider with any medical questions or concerns. Use of this information is at your own risk, and we are not liable for any outcomes resulting from its use.